|
Comment: Compound 20 is reported in a medicinal chemistry study to identify inhibitors of the HCV NS3 protease [ 1]. Compound 20 is a peptide boronic acid containing an extended, hydrophobic P1 residue. The boronic acid/P1 moeity is ligated to the small peptide H-Asp-Glu-Val-Val-Pro. The compound also inhibits human chymotrypsin C ( CTRC).
|
|
2D Structure
|
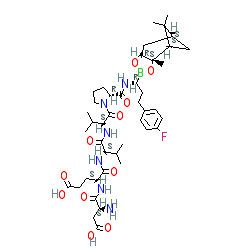
|
Physico-chemical Properties
|
|
|
Hydrogen bond acceptors
|
17
|
|
Hydrogen bond donors
|
7
|
|
Rotatable bonds
|
25
|
|
Topological polar surface area
|
255.79
|
|
Molecular weight
|
870.47
|
|
XLogP
|
2.99
|
|
No. Lipinski's rules broken
|
3
|
|
SMILES / InChI / InChIKey
|
|
|
Canonical SMILES
|
OC(=O)CCC(C(=O)NC(C(=O)NC(C(=O)N1CCCC1C(=O)NC(B1OC2C(O1)(C)C1CC(C2)C1(C)C)CCc1ccc(cc1)F)C(C)C)C(C)C)NC(=O)C(CC(=O)O)N
|
|
Isomeric SMILES
|
OC(=O)CC[C@@H](C(=O)N[C@H](C(=O)N[C@H](C(=O)N1CCC[C@@H]1C(=O)N[C@H](B1O[C@H]2[C@](O1)(C)[C@H]1C[C@@H](C2)C1(C)C)CCc1ccc(cc1)F)C(C)C)C(C)C)NC(=O)[C@H](CC(=O)O)N
|
|
InChI
|
InChI=1S/C43H64BFN6O11/c1-22(2)35(49-38(57)28(15-17-33(52)53)47-37(56)27(46)21-34(54)55)40(59)50-36(23(3)4)41(60)51-18-8-9-29(51)39(58)48-32(16-12-24-10-13-26(45)14-11-24)44-61-31-20-25-19-30(42(25,5)6)43(31,7)62-44/h10-11,13-14,22-23,25,27-32,35-36H,8-9,12,15-21,46H2,1-7H3,(H,47,56)(H,48,58)(H,49,57)(H,50,59)(H,52,53)(H,54,55)/t25-,27-,28-,29+,30-,31+,32-,35-,36-,43-/m0/s1
|
|
InChI Key
|
JAXFJECJQZDFJS-XHEPKHHKSA-N
|
|
|



