|
Comment: There is some ambiguity in the literature and on other online resources as to the exact structure and stereochemistry of etorphine. The structure shown here does not specify stereochemistry and corresponds to the PubChem entry linked to above. Activity data in PubChem is distributed across several CIDs representing slight chiral variations in the molecule. Please note that the ChEBI and ChEMBL entries linked to above do specify stereochemistry and represent the structure differently to us.
|
|
2D Structure
|
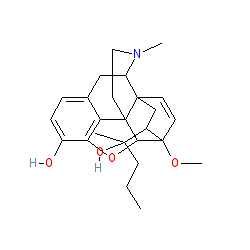
|
Physico-chemical Properties
|
|
|
Hydrogen bond acceptors
|
3
|
|
Hydrogen bond donors
|
2
|
|
Rotatable bonds
|
4
|
|
Topological polar surface area
|
62.16
|
|
Molecular weight
|
411.24
|
|
XLogP
|
1.95
|
|
No. Lipinski's rules broken
|
0
|
|
SMILES / InChI / InChIKey
|
|
|
Canonical SMILES
|
CCCC(C1CC23C=CC1(OC)C1C43CCN(C2Cc2c4c(O1)c(O)cc2)C)(O)C
|
|
Isomeric SMILES
|
CCCC(C1CC23C=CC1(OC)C1C43CCN(C2Cc2c4c(O1)c(O)cc2)C)(O)C
|
|
InChI
|
InChI=1S/C25H33NO4/c1-5-8-22(2,28)17-14-23-9-10-25(17,29-4)21-24(23)11-12-26(3)18(23)13-15-6-7-16(27)20(30-21)19(15)24/h6-7,9-10,17-18,21,27-28H,5,8,11-14H2,1-4H3
|
|
InChI Key
|
CAHCBJPUTCKATP-UHFFFAOYSA-N
|
|
|

