|
|
|
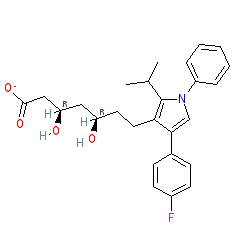
2D Structure
|
|
Physico-chemical Properties
|
|
|
Hydrogen bond acceptors
|
4
|
|
Hydrogen bond donors
|
2
|
|
Rotatable bonds
|
10
|
|
Topological polar surface area
|
85
|
|
Molecular weight
|
438.21
|
|
XLogP
|
3.93
|
|
No. Lipinski's rules broken
|
0
|
|
SMILES / InChI / InChIKey
|
|
|
Canonical SMILES
|
OC(CC(CC(=O)[O-])O)CCc1c(cn(c1C(C)C)c1ccccc1)c1ccc(cc1)F
|
|
Isomeric SMILES
|
O[C@@H](C[C@H](CC(=O)[O-])O)CCc1c(cn(c1C(C)C)c1ccccc1)c1ccc(cc1)F
|
|
InChI
|
InChI=1S/C26H30FNO4/c1-17(2)26-23(13-12-21(29)14-22(30)15-25(31)32)24(18-8-10-19(27)11-9-18)16-28(26)20-6-4-3-5-7-20/h3-11,16-17,21-22,29-30H,12-15H2,1-2H3,(H,31,32)/p-1/t21-,22-/m1/s1
|
|
InChI Key
|
UBFHWBHHCSSZAB-FGZHOGPDSA-M
|
|
|

