
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 443 | 11q23.2 | DRD2 | dopamine receptor D2 | 9,45 |
| Mouse | 7 | 444 | 9 26.72 cM | Drd2 | dopamine receptor D2 | 88 |
| Rat | 7 | 444 | 8q23 | Drd2 | dopamine receptor D2 | 9,24 |
| Gene and Protein Information Comments | ||||||
| The human D2 receptor exists as two alternatively spliced isoforms [44]. The 443 amino acid receptor is the long form (D2L). The short form (D2S) is 414 amino acids long. | ||||||
Previous and Unofficial Names  |
| D2A and D2B | dopamine D2 receptor | dopamine receptor 2 | D2 receptor | D2R | D2(415) and D2(444) |
Database Links  |
|
| Specialist databases | |
| GPCRdb | drd2_human (Hs), drd2_mouse (Mm), drd2_rat (Rn) |
| Other databases | |
| Alphafold | P14416 (Hs), P61168 (Mm), P61169 (Rn) |
| ChEMBL Target | CHEMBL217 (Hs), CHEMBL3427 (Mm), CHEMBL339 (Rn) |
| DrugBank Target | P14416 (Hs) |
| Ensembl Gene | ENSG00000149295 (Hs), ENSMUSG00000032259 (Mm), ENSRNOG00000008428 (Rn) |
| Entrez Gene | 1813 (Hs), 13489 (Mm), 24318 (Rn) |
| Human Protein Atlas | ENSG00000149295 (Hs) |
| KEGG Gene | hsa:1813 (Hs), mmu:13489 (Mm), rno:24318 (Rn) |
| OMIM | 126450 (Hs) |
| Orphanet | ORPHA121177 (Hs) |
| Pharos | P14416 (Hs) |
| RefSeq Nucleotide | NM_000795 (Hs), NM_010077 (Mm), NM_012547 (Rn) |
| RefSeq Protein | NP_000786 (Hs), NP_034207 (Mm), NP_036679 (Rn) |
| UniProtKB | P14416 (Hs), P61168 (Mm), P61169 (Rn) |
| Wikipedia | DRD2 (Hs) |
Selected 3D Structures  |
|||||||||||||
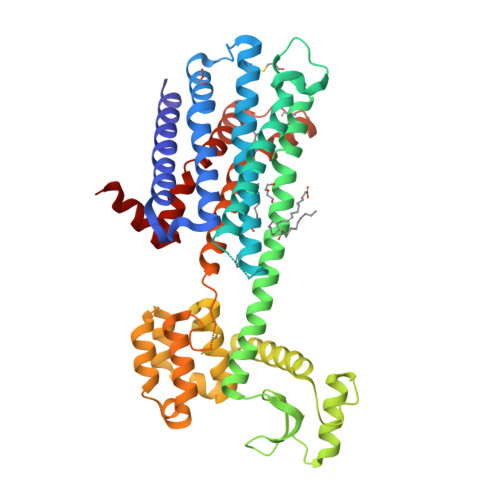
|
|
||||||||||||
Natural/Endogenous Ligands  |
| dopamine |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific agonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Terguride and roxindole have been reported to be partial agonists at the D2S receptor and antagonists at the D2L receptor. Although benzquinamide has higher affinity for α-adrenoceptors, it is suggested in [46] that it is more likely that drug-induced modulation of D2 receptor activity is responsible for the drug's antiemetic action. Allosteric modulation of the D2 receptor by SB269652 only occurs when D2 receptor dimers form, with the ligand assuming a 'bitopic' pose and interacting with different sites on each of the two protomers in the dimer [70]. UNC0006, UNC9994 and UNC9975 are partial biased agonists of β-arrestin 2 recruitment at the D2 receptor as measured using three different assays (β-arrestin-2 translocation Tango assay, DiscoveRx assay and BRET-based β-arrestin-2 recruitment assay) [3]. Tango assay pEC50 values and binding Kis are provided in the table above. G protein-biased, cariprazine-based partial agonists are reported in [115]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Terguride/roxindole have been reported to act as partial agonists at the D2S receptor and as antagonists at the D2L receptor. Perphenazine is an antagonist at both the D2S and D2L receptors [124]. The approved drug mesoridazine, although consisting of 4 stereoisomers, appears to be selective for the D2 receptor [32], especially when examining the data for the two highest affinity isomers, compounds 2 and 5. Across the 3 dopamine receptors, compounds 2 and 5 have the same order of potency (D2>D3>D1). The data shown in the table above is for compound 2. Mesoridazine is also a selective antagonist of the serotonin 5-HT2A receptor. Zotepine has a Ki of 5.4nM for the D2S receptor isoform [107]. The β-arrestin biased ligands UNC9975, UNC0006 and UNC9994, do not activate D2 receptor-mediated Gi-regulated inhibition of cAMP production, but rather are functionally-selective antagonists of the interaction between D2 receptor and β-arrestin-2 [3]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulators | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| D2 receptor-mediated anti-inflammatory effects in the kidney have a protective effect. In contrast, impaired D2 receptor function results in renal inflammation and organ damage [139]. Taken together with evidence that Drd2 knockout mice have a phenotype that includes a profound brain inflammation, these findings suggest that the dopaminergic system acts as an endogenous, evolutionarily conserved, anti-inflammatory mechanism. Evidence from Han et al. (2017) [48] indicates that D2 receptor-induced anti-inflammatory effects in acute pancreatitis models are mediated via a protein phosphatase 2 (PP2A)-dependent Akt/NF-κB pathway. |
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
|
Gi/Go family G protein independent mechanism |
Adenylyl cyclase inhibition Other - See Comments |
|
Comments:
Dopamine D2 receptors can inhibit the Akt (protein kinase B) pathway through a β-arrestin 2/Akt/protein phosphatase 2A complex [15-16]. D2L-induced inhibition of Akt is reported in pituitary lactotrophs, with D2S-induced ERK stimulation also reported in these cells [54,95]. |
|
| References: 56,89,91 | |
Secondary Transduction Mechanisms  |
|
| Transducer | Effector/Response |
|
Potassium channel Other - See Comments |
|
| Comments: β-arrestin recruitment [40,84,135-136]. | |
| References: 31 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||
|
||||||||||
|
||||||||||
Biologically Significant Variants 
|
||||||||
|
||||||||
|
||||||||
|
| General Comments |
| The D2 receptor exists as two alternatively spliced isoforms [44], D2L and D2S. |
1. Agai-Csongor E, Domány G, Nógrádi K, Galambos J, Vágó I, Keserű GM, Greiner I, Laszlovszky I, Gere A, Schmidt E et al.. (2012) Discovery of cariprazine (RGH-188): a novel antipsychotic acting on dopamine D3/D2 receptors. Bioorg Med Chem Lett, 22 (10): 3437-40. [PMID:22537450]
2. Albert PR, Neve KA, Bunzow JR, Civelli O. (1990) Coupling of a cloned rat dopamine-D2 receptor to inhibition of adenylyl cyclase and prolactin secretion. J Biol Chem, 265 (4): 2098-104. [PMID:1688845]
3. Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, Peterson S, Yadav PN, Huang XP, Feng B et al.. (2011) Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci USA, 108 (45): 18488-93. [PMID:22025698]
4. Ambrosio C, Stefanini E. (1991) Interaction of flunarizine with dopamine D2 and D1 receptors. Eur J Pharmacol, 197 (2-3): 221-3. [PMID:1833208]
5. Amenta F, Chiandussi L, Mancini M, Ricci A, Schena M, Veglio F. (1994) Pharmacological characterization and autoradiographic localization of dopamine receptors in the human adrenal cortex. Eur J Endocrinol, 131 (1): 91-6. [PMID:8038912]
6. Amenta F, Collier WL, Ricci A. (1990) Autoradiographic localization of vascular dopamine receptors. Am J Hypertens, 3 (6 Pt 2): 34S-36S. [PMID:2200435]
7. Amenta F, Ricci A, Vega JA. (1990) Pharmacological characterization of rat renal medulla dopamine-sensitive cyclic adenosine monophosphate generating system. J Pharmacol Exp Ther, 253 (1): 246-9. [PMID:2158544]
8. An JJ, Bae MH, Cho SR, Lee SH, Choi SH, Lee BH, Shin HS, Kim YN, Park KW, Borrelli E et al.. (2004) Altered GABAergic neurotransmission in mice lacking dopamine D2 receptors. Mol Cell Neurosci, 25 (4): 732-41. [PMID:15080900]
9. Araki K, Kuwano R, Morii K, Hayashi S, Minoshima S, Shimizu N, Katagiri T, Usui H, Kumanishi T, Takahashi Y. (1992) Structure and expression of human and rat D2 dopamine receptor genes. Neurochem Int, 21 (1): 91-8. [PMID:1363862]
10. Ariano MA, Stromski CJ, Smyk-Randall EM, Sibley DR. (1992) D2 dopamine receptor localization on striatonigral neurons. Neurosci Lett, 144 (1-2): 215-20. [PMID:1436705]
11. Arnt J, Skarsfeldt T. (1998) Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology, 18 (2): 63-101. [PMID:9430133]
12. Asa SL, Kelly MA, Grandy DK, Low MJ. (1999) Pituitary lactotroph adenomas develop after prolonged lactotroph hyperplasia in dopamine D2 receptor-deficient mice. Endocrinology, 140 (11): 5348-55. [PMID:10537166]
13. Auerbach SS, DrugMatrix® and ToxFX® Coordinator National Toxicology Program. National Toxicology Program: Dept of Health and Human Services. Accessed on 02/05/2014. Modified on 02/05/2014. DrugMatrix, https://ntp.niehs.nih.gov/drugmatrix/index.html
14. Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. (1995) Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature, 377 (6548): 424-8. [PMID:7566118]
15. Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. (2005) An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell, 122 (2): 261-73. [PMID:16051150]
16. Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. (2007) Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci, 27 (4): 881-5. [PMID:17251429]
17. Benoit-Marand M, Borrelli E, Gonon F. (2001) Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci, 21 (23): 9134-41. [PMID:11717346]
18. Bertorello A, Aperia A. (1990) Inhibition of proximal tubule Na(+)-K(+)-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am J Physiol, 259 (6 Pt 2): F924-8. [PMID:1979719]
19. Bertorello AM, Hopfield JF, Aperia A, Greengard P. (1990) Inhibition by dopamine of (Na(+)+K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature, 347: 386-388. [PMID:1977083]
20. Boulay D, Depoortere R, Oblin A, Sanger DJ, Schoemaker H, Perrault G. (2000) Haloperidol-induced catalepsy is absent in dopamine D(2), but maintained in dopamine D(3) receptor knock-out mice. Eur J Pharmacol, 391 (1-2): 63-73. [PMID:10720636]
21. Boulay D, Depoortere R, Perrault G, Borrelli E, Sanger DJ. (1999) Dopamine D2 receptor knock-out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology, 38: 1389-1396. [PMID:10471093]
22. Breese GR, Duncan GE, Napier TC, Bondy SC, Iorio LC, Mueller RA. (1987) 6-hydroxydopamine treatments enhance behavioral responses to intracerebral microinjection of D1- and D2-dopamine agonists into nucleus accumbens and striatum without changing dopamine antagonist binding. J Pharmacol Exp Ther, 240 (1): 167-76. [PMID:3100767]
23. Bughi S, Jost-Vu E, Antonipillai I, Nadler J, Horton R. (1994) Effect of dopamine2 blockade on renal function under varied sodium intake. J Clin Endocrinol Metab, 78 (5): 1079-84. [PMID:8175964]
24. Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. (1988) Cloning and expression of a rat D2 dopamine receptor cDNA. Nature, 336 (6201): 783-7. [PMID:2974511]
25. Burris KD, Pacheco MA, Filtz TM, Kung MP, Kung HF, Molinoff PB. (1995) Lack of discrimination by agonists for D2 and D3 dopamine receptors. Neuropsychopharmacology, 12 (4): 335-45. [PMID:7576010]
26. Burstein ES, Ma J, Wong S, Gao Y, Pham E, Knapp AE, Nash NR, Olsson R, Davis RE, Hacksell U et al.. (2005) Intrinsic efficacy of antipsychotics at human D2, D3, and D4 dopamine receptors: identification of the clozapine metabolite N-desmethylclozapine as a D2/D3 partial agonist. J Pharmacol Exp Ther, 315 (3): 1278-87. [PMID:16135699]
27. Cadet JL, Zhu SM, Angulo JA. (1992) Quantitative in situ hybridization evidence for differential regulation of proenkephalin and dopamine D2 receptor mRNA levels in the rat striatum: effects of unilateral intrastriatal injections of 6-hydroxydopamine. Brain Res Mol Brain Res, 12 (1-3): 59-67. [PMID:1312206]
28. Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. (2002) Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci, 22 (7): 2977-88. [PMID:11923462]
29. Calabresi P, Saiardi A, Pisani A, Baik JH, Centonze D, Mercuri NB, Bernardi G, Borrelli E. (1997) Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J Neurosci, 17 (12): 4536-44. [PMID:9169514]
30. Castellano MA, Liu LX, Monsma Jr FJ, Sibley DR, Kapatos G, Chiodo LA. (1993) Transfected D2 short dopamine receptors inhibit voltage-dependent potassium current in neuroblastoma x glioma hybrid (NG108-15) cells. Mol Pharmacol, 44 (3): 649-56. [PMID:8371717]
31. Castelletti L, Memo M, Missale C, Spano PF, Valerio A. (1989) Potassium channels involved in the transduction mechanism of dopamine D2 receptors in rat lactotrophs. J Physiol (Lond.), 410: 251-65. [PMID:2552081]
32. Choi S, Haggart D, Toll L, Cuny GD. (2004) Synthesis, receptor binding and functional studies of mesoridazine stereoisomers. Bioorg Med Chem Lett, 14 (17): 4379-82. [PMID:15357957]
33. Chumpradit S, Kung MP, Vessotskie J, Foulon C, Mu M, Kung HF. (1994) Iodinated 2-aminotetralins and 3-amino-1-benzopyrans: ligands for dopamine D2 and D3 receptors. J Med Chem, 37 (24): 4245-50. [PMID:7990123]
34. Cook CC, Gurling HM. (1994) The D2 dopamine receptor gene and alcoholism: a genetic effect in the liability for alcoholism. J R Soc Med, 87 (7): 400-2. [PMID:8046727]
35. Cunningham CL, Howard MA, Gill SJ, Rubinstein M, Low MJ, Grandy DK. (2000) Ethanol-conditioned place preference is reduced in dopamine D2 receptor-deficient mice. Pharmacol Biochem Behav, 67 (4): 693-9. [PMID:11166059]
36. Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. (1999) Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem, 72 (1): 148-56. [PMID:9886065]
37. Dijkstra D, Rodenhuis N, Vermeulen ES, Pugsley TA, Wise LD, Wikström HV. (2002) Further characterization of structural requirements for ligands at the dopamine D(2) and D(3) receptor: exploring the thiophene moiety. J Med Chem, 45 (14): 3022-31. [PMID:12086487]
38. Elmer GI, Pieper JO, Levy J, Rubinstein M, Low MJ, Grandy DK, Wise RA. (2005) Brain stimulation and morphine reward deficits in dopamine D2 receptor-deficient mice. Psychopharmacology (Berl.), 182 (1): 33-44. [PMID:16136297]
39. Fowler SC, Zarcone TJ, Vorontsova E, Chen R. (2002) Motor and associative deficits in D2 dopamine receptor knockout mice. Int J Dev Neurosci, 20 (3-5): 309-21. [PMID:12175868]
40. Free RB, Chun LS, Moritz AE, Miller BN, Doyle TB, Conroy JL, Padron A, Meade JA, Xiao J, Hu X et al.. (2014) Discovery and characterization of a G protein-biased agonist that inhibits β-arrestin recruitment to the D2 dopamine receptor. Mol Pharmacol, 86 (1): 96-105. [PMID:24755247]
41. Freedman SB, Patel S, Marwood R, Emms F, Seabrook GR, Knowles MR, McAllister G. (1994) Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther, 268 (1): 417-26. [PMID:8301582]
42. Gacsályi I, Nagy K, Pallagi K, Lévay G, Hársing Jr LG, Móricz K, Kertész S, Varga P, Haller J, Gigler G et al.. (2013) Egis-11150: a candidate antipsychotic compound with procognitive efficacy in rodents. Neuropharmacology, 64: 254-63. [PMID:22824189]
43. Gandelman KY, Harmon S, Todd RD, O'Malley KL. (1991) Analysis of the structure and expression of the human dopamine D2A receptor gene. J Neurochem, 56 (3): 1024-9. [PMID:1825222]
44. Giros B, Sokoloff P, Martres MP, Riou JF, Emorine LJ, Schwartz JC. (1989) Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature, 342 (6252): 923-6. [PMID:2531847]
45. Grandy DK, Marchionni MA, Makam H, Stofko RE, Alfano M, Frothingham L, Fischer JB, Burke-Howie KJ, Bunzow JR, Server AC et al.. (1989) Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci USA, 86 (24): 9762-6. [PMID:2532362]
46. Gregori-Puigjané E, Setola V, Hert J, Crews BA, Irwin JJ, Lounkine E, Marnett L, Roth BL, Shoichet BK. (2012) Identifying mechanism-of-action targets for drugs and probes. Proc Natl Acad Sci USA, 109 (28): 11178-83. [PMID:22711801]
47. Grundt P, Husband SL, Luedtke RR, Taylor M, Newman AH. (2007) Analogues of the dopamine D2 receptor antagonist L741,626: Binding, function, and SAR. Bioorg Med Chem Lett, 17 (3): 745-9. [PMID:17095222]
48. Han X, Li B, Ye X, Mulatibieke T, Wu J, Dai J, Wu D, Ni J, Zhang R, Xue J et al.. (2017) Dopamine D2 receptor signalling controls inflammation in acute pancreatitis via a PP2A-dependent Akt/NF-κB signalling pathway. Br J Pharmacol, 174 (24): 4751-4770. [PMID:28963856]
49. Heier RF, Dolak LA, Duncan JN, Hyslop DK, Lipton MF, Martin IJ, Mauragis MA, Piercey MF, Nichols NF, Schreur PJ et al.. (1997) Synthesis and biological activities of (R)-5,6-dihydro-N,N-dimethyl-4H-imidazo[4,5,1-ij]quinolin-5-amine and its metabolites. J Med Chem, 40 (5): 639-46. [PMID:9057850]
50. Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suárez-Fariñas M, Schwarz C, Stephan DA, Surmeier DJ et al.. (2008) A translational profiling approach for the molecular characterization of CNS cell types. Cell, 135 (4): 738-48. [PMID:19013281]
51. Heinrich T, Böttcher H, Gericke R, Bartoszyk GD, Anzali S, Seyfried CA, Greiner HE, Van Amsterdam C. (2004) Synthesis and structure--activity relationship in a class of indolebutylpiperazines as dual 5-HT(1A) receptor agonists and serotonin reuptake inhibitors. J Med Chem, 47 (19): 4684-92. [PMID:15341484]
52. Hoare SR, Coldwell MC, Armstrong D, Strange PG. (2000) Regulation of human D(1), d(2(long)), d(2(short)), D(3) and D(4) dopamine receptors by amiloride and amiloride analogues. Br J Pharmacol, 130 (5): 1045-59. [PMID:10882389]
53. Hurd YL, Suzuki M, Sedvall GC. (2001) D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J Chem Neuroanat, 22 (1-2): 127-37. [PMID:11470560]
54. Iaccarino C, Samad TA, Mathis C, Kercret H, Picetti R, Borrelli E. (2002) Control of lactotrop proliferation by dopamine: essential role of signaling through D2 receptors and ERKs. Proc Natl Acad Sci USA, 99 (22): 14530-5. [PMID:12391292]
55. Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y, Toma S et al.. (2010) Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther, 334 (1): 171-81. [PMID:20404009]
56. Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. (2001) Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc Natl Acad Sci USA, 98 (6): 3577-82. [PMID:11248120]
57. Jordan CJ, Humburg B, Rice M, Bi GH, You ZB, Shaik AB, Cao J, Bonifazi A, Gadiano A, Rais R et al.. (2019) The highly selective dopamine D3R antagonist, R-VK4-40 attenuates oxycodone reward and augments analgesia in rodents. Neuropharmacology, 158: 107597. [PMID:30974107]
58. Kariya S, Isozaki S, Masubuchi Y, Suzuki T, Narimatsu S. (1995) Possible pharmacokinetic and pharmacodynamic factors affecting parkinsonism inducement by cinnarizine and flunarizine. Biochem Pharmacol, 50 (10): 1645-50. [PMID:7503767]
59. Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, Allen RG, Hnasko R, Ben-Jonathan N, Grandy DK, Low MJ. (1997) Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron, 19: 103-113. [PMID:9247267]
60. Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow J R, Fang Y, Gerhardt GA, Grandy DK, Low MJ. (1998) Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci, 18: 3470-3479. [PMID:9547254]
61. Khlghatyan J, Quintana C, Parent M, Beaulieu JM. (2019) High Sensitivity Mapping of Cortical Dopamine D2 Receptor Expressing Neurons. Cereb Cortex, 29 (9): 3813-3827. [PMID:30295716]
62. Kita JM, Parker LE, Phillips PE, Garris PA, Wightman RM. (2007) Paradoxical modulation of short-term facilitation of dopamine release by dopamine autoreceptors. J Neurochem, 102 (4): 1115-24. [PMID:17663751]
63. Kongsamut S, Kang J, Chen XL, Roehr J, Rampe D. (2002) A comparison of the receptor binding and HERG channel affinities for a series of antipsychotic drugs. Eur J Pharmacol, 450 (1): 37-41. [PMID:12176106]
64. Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL. (2003) H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology, 28 (3): 519-26. [PMID:12629531]
65. Kukreti R, Tripathi S, Bhatnagar P, Gupta S, Chauhan C, Kubendran S, Janardhan Reddy YC, Jain S, Brahmachari SK. (2006) Association of DRD2 gene variant with schizophrenia. Neurosci Lett, 392 (1-2): 68-71. [PMID:16183199]
66. Kulagowski JJ, Broughton HB, Curtis NR, Mawer IM, Ridgill MP, Baker R, Emms F, Freedman SB, Marwood R, Patel S et al.. (1996) 3-((4-(4-Chlorophenyl)piperazin-1-yl)-methyl)-1H-pyrrolo-2,3-b-pyridine: an antagonist with high affinity and selectivity for the human dopamine D4 receptor. J Med Chem, 39 (10): 1941-2. [PMID:8642550]
67. Köhler C, Hall H, Ogren SO, Gawell L. (1985) Specific in vitro and in vivo binding of 3H-raclopride. A potent substituted benzamide drug with high affinity for dopamine D-2 receptors in the rat brain. Biochem Pharmacol, 34 (13): 2251-9. [PMID:4015674]
68. L'hirondel M, Chéramy A, Godeheu G, Artaud F, Saiardi A, Borrelli E, Glowinski J. (1998) Lack of autoreceptor-mediated inhibitory control of dopamine release in striatal synaptosomes of D2 receptor-deficient mice. Brain Res, 792 (2): 253-62. [PMID:9593923]
69. Lajiness ME, Chio CL, Huff RM. (1993) D2 dopamine receptor stimulation of mitogenesis in transfected Chinese hamster ovary cells: relationship to dopamine stimulation of tyrosine phosphorylations. J Pharmacol Exp Ther, 267 (3): 1573-81. [PMID:7903393]
70. Lane JR, Donthamsetti P, Shonberg J, Draper-Joyce CJ, Dentry S, Michino M, Shi L, López L, Scammells PJ, Capuano B et al.. (2014) A new mechanism of allostery in a G protein-coupled receptor dimer. Nat Chem Biol, 10 (9): 745-52. [PMID:25108820]
71. Leopoldo M, Lacivita E, Contino M, Colabufo NA, Berardi F, Perrone R. (2007) Structure-activity relationship study on N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides, a class of 5-HT7 receptor agents. 2. J Med Chem, 50 (17): 4214-21. [PMID:17649988]
72. Leopoldo M, Lacivita E, De Giorgio P, Fracasso C, Guzzetti S, Caccia S, Contino M, Colabufo NA, Berardi F, Perrone R. (2008) Structural modifications of N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides: influence on lipophilicity and 5-HT7 receptor activity. Part III. J Med Chem, 51 (18): 5813-22. [PMID:18800769]
73. Li P, Zhang Q, Robichaud AJ, Lee T, Tomesch J, Yao W, Beard JD, Snyder GL, Zhu H, Peng Y et al.. (2014) Discovery of a tetracyclic quinoxaline derivative as a potent and orally active multifunctional drug candidate for the treatment of neuropsychiatric and neurological disorders. J Med Chem, 57 (6): 2670-82. [PMID:24559051]
74. Liu YF, Civelli O, Grandy DK, Albert PR. (1992) Differential sensitivity of the short and long human dopamine D2 receptor subtypes to protein kinase C. J Neurochem, 59 (6): 2311-7. [PMID:1331329]
75. MacKenzie RG, VanLeeuwen D, Pugsley TA, Shih YH, Demattos S, Tang L, Todd RD, O'Malley KL. (1994) Characterization of the human dopamine D3 receptor expressed in transfected cell lines. Eur J Pharmacol, 266 (1): 79-85. [PMID:7907989]
76. Maeda K, Sugino H, Akazawa H, Amada N, Shimada J, Futamura T, Yamashita H, Ito N, McQuade RD, Mørk A et al.. (2014) Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther, 350 (3): 589-604. [PMID:24947465]
77. Maggio R, Scarselli M, Novi F, Millan MJ, Corsini GU. (2003) Potent activation of dopamine D3/D2 heterodimers by the antiparkinsonian agents, S32504, pramipexole and ropinirole. J Neurochem, 87 (3): 631-41. [PMID:14535946]
78. Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E. (1997) Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature, 388 (6642): 586-9. [PMID:9252189]
79. Malmberg A, Jackson DM, Eriksson A, Mohell N. (1993) Unique binding characteristics of antipsychotic agents interacting with human dopamine D2A, D2B, and D3 receptors. Mol Pharmacol, 43 (5): 749-54. [PMID:8099194]
80. Manzanedo C, Aguilar MA, Rodríguez-Arias M, Miñarro J. (2005) Sensitization to the rewarding effects of morphine depends on dopamine. Neuroreport, 16 (2): 201-5. [PMID:15671878]
81. Matsui A, Matsuo H, Takanaga H, Sasaki S, Maeda M, Sawada Y. (1998) Prediction of catalepsies induced by amiodarone, aprindine and procaine: similarity in conformation of diethylaminoethyl side chain. J Pharmacol Exp Ther, 287 (2): 725-32. [PMID:9808703]
82. McAllister G, Knowles MR, Ward-Booth SM, Sinclair HA, Patel S, Marwood R, Emms F, Patel S, Smith A, Seabrook GR et al.. (1995) Functional coupling of human D2, D3, and D4 dopamine receptors in HEK293 cells. J Recept Signal Transduct Res, 15 (1-4): 267-81. [PMID:8903944]
83. McCall RB, Lookingland KJ, Bédard PJ, Huff RM. (2005) Sumanirole, a highly dopamine D2-selective receptor agonist: in vitro and in vivo pharmacological characterization and efficacy in animal models of Parkinson's disease. J Pharmacol Exp Ther, 314 (3): 1248-56. [PMID:15980060]
84. Meade JA, Free RB, Miller NR, Chun LS, Doyle TB, Moritz AE, Conroy JL, Watts VJ, Sibley DR. (2015) (-)-Stepholidine is a potent pan-dopamine receptor antagonist of both G protein- and β-arrestin-mediated signaling. Psychopharmacology (Berl.), 232 (5): 917-30. [PMID:25231919]
85. Mierau J, Schneider FJ, Ensinger HA, Chio CL, Lajiness ME, Huff RM. (1995) Pramipexole binding and activation of cloned and expressed dopamine D2, D3 and D4 receptors. Eur J Pharmacol, 290 (1): 29-36. [PMID:7664822]
86. Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. (2002) Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther, 303 (2): 791-804. [PMID:12388666]
87. Millan MJ, Peglion JL, Vian J, Rivet JM, Brocco M, Gobert A, Newman-Tancredi A, Dacquet C, Bervoets K, Girardon S. (1995) Functional correlates of dopamine D3 receptor activation in the rat in vivo and their modulation by the selective antagonist, (+)-S 14297: 1. Activation of postsynaptic D3 receptors mediates hypothermia, whereas blockade of D2 receptors elicits prolactin secretion and catalepsy. J Pharmacol Exp Ther, 275: 885-898. [PMID:7473180]
88. Montmayeur JP, Bausero P, Amlaiky N, Maroteaux L, Hen R, Borrelli E. (1991) Differential expression of the mouse D2 dopamine receptor isoforms. FEBS Lett, 278 (2): 239-43. [PMID:1991517]
89. Montmayeur JP, Borrelli E. (1991) Transcription mediated by a cAMP-responsive promoter element is reduced upon activation of dopamine D2 receptors. Proc Natl Acad Sci USA, 88 (8): 3135-9. [PMID:1849644]
90. Ochi T, Sakamoto M, Minamida A, Suzuki K, Ueda T, Une T, Toda H, Matsumoto K, Terauchi Y. (2005) Syntheses and properties of the major hydroxy metabolites in humans of blonanserin AD-5423, a novel antipsychotic agent. Bioorg Med Chem Lett, 15 (4): 1055-9. [PMID:15686911]
91. Onali P, Schwartz JP. (1983) Inhibition of VIP-sensitive adenylate cyclase by dopamine in rat anterior pituitary. Adv Biochem Psychopharmacol, 36: 199-207. [PMID:6344565]
92. Patel S, Patel S, Marwood R, Emms F, Marston D, Leeson PD, Curtis NR, Kulagowski JJ, Freedman SB. (1996) Identification and pharmacological characterization of [125I]L-750,667, a novel radioligand for the dopamine D4 receptor. Mol Pharmacol, 50 (6): 1658-64. [PMID:8967990]
93. Pillai G, Brown NA, McAllister G, Milligan G, Seabrook GR. (1998) Human D2 and D4 dopamine receptors couple through betagamma G-protein subunits to inwardly rectifying K+ channels (GIRK1) in a Xenopus oocyte expression system: selective antagonism by L-741,626 and L-745,870 respectively. Neuropharmacology, 37: 983-987. [PMID:9833627]
94. Pugsley TA, Davis MD, Akunne HC, MacKenzie RG, Shih YH, Damsma G, Wikstrom H, Whetzel SZ, Georgic LM, Cooke LW et al.. (1995) Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. J Pharmacol Exp Ther, 275 (3): 1355-66. [PMID:8531103]
95. Radl D, De Mei C, Chen E, Lee H, Borrelli E. (2013) Each individual isoform of the dopamine D2 receptor protects from lactotroph hyperplasia. Mol Endocrinol, 27 (6): 953-65. [PMID:23608643]
96. Ralph RJ, Varty GB, Kelly MA, Wang YM, Caron MG, Rubinstein M, Grandy DK, Low MJ, Geyer MA. (1999) The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J Neurosci, 19 (11): 4627-33. [PMID:10341260]
97. Rocchetti J, Isingrini E, Dal Bo G, Sagheby S, Menegaux A, Tronche F, Levesque D, Moquin L, Gratton A, Wong TP et al.. (2015) Presynaptic D2 dopamine receptors control long-term depression expression and memory processes in the temporal hippocampus. Biol Psychiatry, 77 (6): 513-25. [PMID:24742619]
98. Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli E. (2002) Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J Neurosci, 22 (8): 3293-301. [PMID:11943831]
99. Saiardi A, Borrelli E. (1998) Absence of dopaminergic control on melanotrophs leads to Cushing's-like syndrome in mice. Mol Endocrinol, 12 (8): 1133-9. [PMID:9717839]
100. Saiardi A, Bozzi Y, Baik JH, Borrelli E. (1997) Antiproliferative role of dopamine: loss of D2 receptors causes hormonal dysfunction and pituitary hyperplasia. Neuron, 19 (1): 115-26. [PMID:9247268]
101. Sasse BC, Mach UR, Leppaenen J, Calmels T, Stark H. (2007) Hybrid approach for the design of highly affine and selective dopamine D(3) receptor ligands using privileged scaffolds of biogenic amine GPCR ligands. Bioorg Med Chem, 15 (23): 7258-73. [PMID:17826096]
102. Sautel F, Griffon N, Lévesque D, Pilon C, Schwartz JC, Sokoloff P. (1995) A functional test identifies dopamine agonists selective for D3versus D2receptors. Neuroreport, 6: 329-332. [PMID:7756621]
103. Sautel F, Griffon N, Sokoloff P, Schwartz JC, Launay C, Simon P, Costentin J, Schoenfelder A, Garrido F, Mann A et al.. (1995) Nafadotride, a potent preferential dopamine D3 receptor antagonist, activates locomotion in rodents. J Pharmacol Exp Ther, 275 (3): 1239-46. [PMID:8531087]
104. Schetz JA, Benjamin PS, Sibley DR. (2000) Nonconserved residues in the second transmembrane-spanning domain of the D(4) dopamine receptor are molecular determinants of D(4)-selective pharmacology. Mol Pharmacol, 57 (1): 144-52. [PMID:10617689]
105. Schinelli S, Paolillo M, Corona GL. (1994) Opposing actions of D1- and D2-dopamine receptors on arachidonic acid release and cyclic AMP production in striatal neurons. J Neurochem, 62 (3): 944-9. [PMID:8113815]
106. Schmitz Y, Schmauss C, Sulzer D. (2002) Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci, 22 (18): 8002-9. [PMID:12223553]
107. Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE. (1996) Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl.), 124 (1-2): 57-73. [PMID:8935801]
108. Seeman P. (2001) Antipsychotic drugs, dopamine receptors, and schizophrenia. Clinical Neuroscience Research, 1 (1-2): 53-60. DOI: 10.1016/S1566-2772(00)00007-4
109. Seeman P, Chau-Wong M, Tedesco J, Wong K. (1975) Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci USA, 72 (11): 4376-80. [PMID:1060115]
110. Seeman P, Corbett R, Van Tol HH. (1997) Atypical neuroleptics have low affinity for dopamine D2 receptors or are selective for D4 receptors. Neuropsychopharmacology, 16 (2): 93-110; discussion 111-35. [PMID:9015795]
111. Seeman P, Tallerico T. (1998) Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry, 3 (2): 123-34. [PMID:9577836]
112. Serý O, Drtílková I, Theiner P, Pitelová R, Staif R, Znojil V, Lochman J, Didden W. (2006) Polymorphism of DRD2 gene and ADHD. Neuro Endocrinol Lett, 27 (1-2): 236-40. [PMID:16648784]
113. Setlow B, McGaugh JL. (2000) D2 dopamine receptor blockade immediately post-training enhances retention in hidden and visible platform versions of the water maze. Learn Mem, 7 (3): 187-91. [PMID:10837508]
114. Shahid M, Walker GB, Zorn SH, Wong EH. (2009) Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol (Oxford), 23 (1): 65-73. [PMID:18308814]
115. Shen Y, McCorvy JD, Martini ML, Rodriguiz RM, Pogorelov VM, Ward KM, Wetsel WC, Liu J, Roth BL, Jin J. (2019) D2 Dopamine Receptor G Protein-Biased Partial Agonists Based on Cariprazine. J Med Chem, 62 (9): 4755-4771. [PMID:30964661]
116. Skaaning Jensen B, Levavi-Sivan B, Fishburn CS, Fuchs S. (1997) Functional expression of the murine D2, D3, and D4 dopamine receptors in Xenopus laevis oocytes. FEBS Lett, 420: 191-195. [PMID:9459308]
117. Snyder GL, Vanover KE, Zhu H, Miller DB, O'Callaghan JP, Tomesch J, Li P, Zhang Q, Krishnan V, Hendrick JP et al.. (2015) Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl.), 232 (3): 605-21. [PMID:25120104]
118. Sokoloff P, Andrieux M, Besançon R, Pilon C, Martres MP, Giros B, Schwartz JC. (1992) Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur J Pharmacol, 225 (4): 331-7. [PMID:1354163]
119. Sokoloff P, Giros B, Martres MP, Andrieux M, Besancon R, Pilon C, Bouthenet ML, Souil E, Schwartz JC. (1992) Localization and function of the D3 dopamine receptor. Arzneimittelforschung, 42 (2A): 224-30. [PMID:1586393]
120. Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. (1990) Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature, 347 (6289): 146-51. [PMID:1975644]
121. Spetea M, Berzetei-Gurske IP, Guerrieri E, Schmidhammer H. (2012) Discovery and pharmacological evaluation of a diphenethylamine derivative (HS665), a highly potent and selective κ opioid receptor agonist. J Med Chem, 55 (22): 10302-6. [PMID:23134120]
122. Starr BS, Starr MS. (1986) Differential effects of dopamine D1 and D2 agonists and antagonists on velocity of movement, rearing and grooming in the mouse. Implications for the roles of D1 and D2 receptors. Neuropharmacology, 25 (5): 455-63. [PMID:3488514]
123. Strupczewski JT, Bordeau KJ, Chiang Y, Glamkowski EJ, Conway PG, Corbett R, Hartman HB, Szewczak MR, Wilmot CA, Helsley GC. (1995) 3-[[(Aryloxy)alkyl]piperidinyl]-1,2-benzisoxazoles as D2/5-HT2 antagonists with potential atypical antipsychotic activity: antipsychotic profile of iloperidone (HP 873). J Med Chem, 38 (7): 1119-31. [PMID:7707315]
124. Tadori Y, Forbes RA, McQuade RD, Kikuchi T. (2011) Functional potencies of dopamine agonists and antagonists at human dopamine D₂ and D₃ receptors. Eur J Pharmacol, 666 (1-3): 43-52. [PMID:21658377]
125. Tang L, Todd RD, Heller A, O'Malley KL. (1994) Pharmacological and functional characterization of D2, D3 and D4 dopamine receptors in fibroblast and dopaminergic cell lines. J Pharmacol Exp Ther, 268 (1): 495-502. [PMID:8301592]
126. Tice MA, Hashemi T, Taylor LA, Duffy RA, McQuade RD. (1994) Characterization of the binding of SCH 39166 to the five cloned dopamine receptor subtypes. Pharmacol Biochem Behav, 49 (3): 567-71. [PMID:7862709]
127. Tran AH, Tamura R, Uwano T, Kobayashi T, Katsuki M, Matsumoto G, Ono T. (2002) Altered accumbens neural response to prediction of reward associated with place in dopamine D2 receptor knockout mice. Proc Natl Acad Sci USA, 99 (13): 8986-91. [PMID:12084937]
128. Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. (2000) Distinct functions of the two isoforms of dopamine D2 receptors. Nature, 408 (6809): 199-203. [PMID:11089973]
129. Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O. (1991) Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature, 350 (6319): 610-4. [PMID:1840645]
130. von Coburg Y, Kottke T, Weizel L, Ligneau X, Stark H. (2009) Potential utility of histamine H3 receptor antagonist pharmacophore in antipsychotics. Bioorg Med Chem Lett, 19 (2): 538-42. [PMID:19091563]
131. Vukhac KL, Sankoorikal EB, Wang Y. (2001) Dopamine D2L receptor- and age-related reduction in offensive aggression. Neuroreport, 12: 1035-1038. [PMID:11303741]
132. Wang S, Che T, Levit A, Shoichet BK, Wacker D, Roth BL. (2018) Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature, 555 (7695): 269-273. [PMID:29466326]
133. Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB. (2000) Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci, 20 (22): 8305-14. [PMID:11069937]
134. Watts VJ, Neve KA. (1996) Sensitization of endogenous and recombinant adenylate cyclase by activation of D2 dopamine receptors. Mol Pharmacol, 50 (4): 966-76. [PMID:8863843]
135. Xiao J, Free RB, Barnaeva E, Conroy J, Doyle T, Bryant-Genevier M, Taylor MK, Southall N, Hu X, Ferrer M et al.. (2010) Discovery, optimization, and characterization of a novel series of dopamine D2 versus D3 receptor selective antagonists. Probe Reports from the NIH Molecular Libraries Program,. [PMID:24260782]
136. Xiao J, Free RB, Barnaeva E, Conroy JL, Doyle T, Miller B, Bryant-Genevier M, Taylor MK, Hu X, Dulcey AE et al.. (2014) Discovery, optimization, and characterization of novel D2 dopamine receptor selective antagonists. J Med Chem, 57 (8): 3450-63. [PMID:24666157]
137. Yamaguchi H, Aiba A, Nakamura K, Nakao K, Sakagami H, Goto K, Kondo H, Katsuki M. (1996) Dopamine D2 receptor plays a critical role in cell proliferation and proopiomelanocortin expression in the pituitary. Genes Cells, 1 (2): 253-68. [PMID:9140068]
138. Zajdel P, Marciniec K, Maślankiewicz A, Grychowska K, Satała G, Duszyńska B, Lenda T, Siwek A, Nowak G, Partyka A et al.. (2013) Antidepressant and antipsychotic activity of new quinoline- and isoquinoline-sulfonamide analogs of aripiprazole targeting serotonin 5-HT₁A/5-HT₂A/5-HT₇ and dopamine D₂/D₃ receptors. Eur J Med Chem, 60: 42-50. [PMID:23279866]
139. Zhang Y, Jiang X, Qin C, Cuevas S, Jose PA, Armando I. (2016) Dopamine D2 receptors' effects on renal inflammation are mediated by regulation of PP2A function. Am J Physiol Renal Physiol, 310 (2): F128-34. [PMID:26290374]
140. Zhang ZW, Burke MW, Calakos N, Beaulieu JM, Vaucher E. (2010) Confocal Analysis of Cholinergic and Dopaminergic Inputs onto Pyramidal Cells in the Prefrontal Cortex of Rodents. Front Neuroanat, 4: 21. [PMID:20589096]
141. Zhen J, Antonio T, Dutta AK, Reith ME. (2010) Concentration of receptor and ligand revisited in a modified receptor binding protocol for high-affinity radioligands: [3H]Spiperone binding to D2 and D3 dopamine receptors. J Neurosci Methods, 188 (1): 32-8. [PMID:20122961]