
Top ▲

 target has curated data in GtoImmuPdb
target has curated data in GtoImmuPdb
Target id: 2156
Nomenclature: phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma
Abbreviated Name: PI3Kγ
Family: Phosphatidylinositol-4,5-bisphosphate 3-kinase family, Phosphatidylinositol kinases
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 1102 | 7q22.3 | PIK3CG | phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma | |
| Mouse | - | 1102 | 12 A3 | Pik3cg | phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma | |
| Rat | - | 1102 | 6 q16 | Pik3cg | phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit gamma | |
Previous and Unofficial Names  |
| p120-PI3K | PI3Kgamma | p110γ/PIK3CG | phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit gamma | phosphatidylinositol-4 |
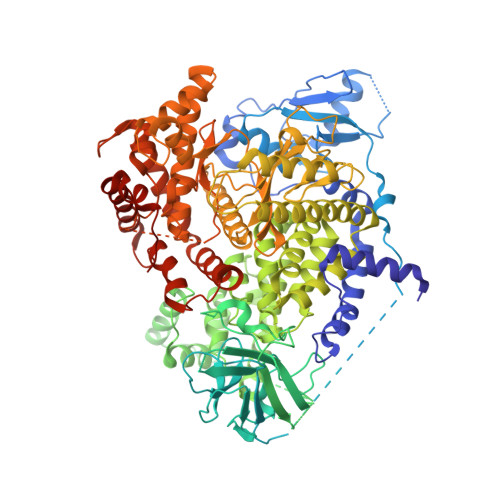
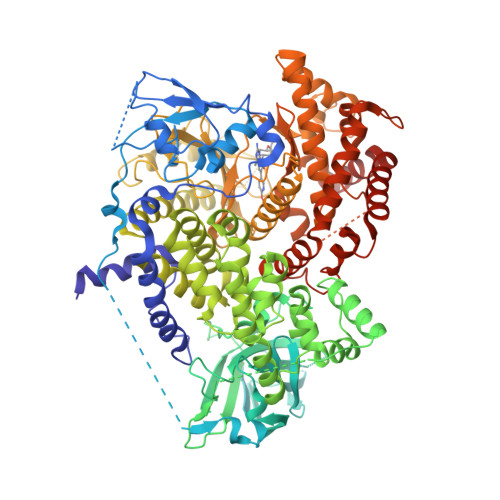
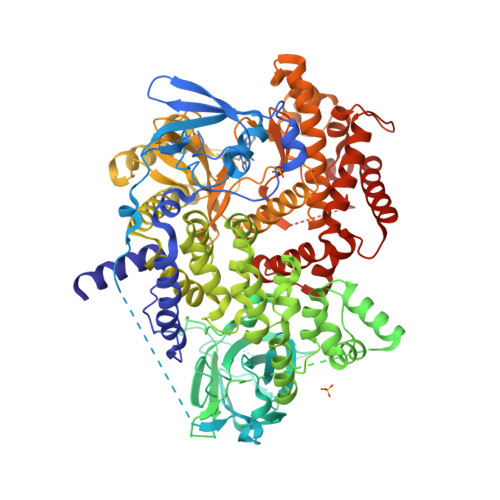
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 18,68 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: PIK3CG | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| The role of PI3Kγ in immuno-oncology is reviewed in [1]. A detailed analysis of the function of PI3Kγ in malignant B cells indicated that it is crucial for chemokine gradient sensing and adhesion to stromal cells (i.e. malignant B cell migration) [2]. The PI3Kγ isoform is of particular therapeutic interest in chronic obstructive airway diseases, such as severe asthma and COPD. This Class IB PI3K isoform has regulatory roles in mediating neutrophil migration (in response to asthmatic epithelial conditioned medium [64]) and degranulation that may contribute to inflammation-mediated tissue damage of the airways [49]. |
| Immuno Process Associations | ||||
|
||||
|
||||
|
||||
|
||||
|
||||
|
||||
|
||||
|
1. Adams JL, Smothers J, Srinivasan R, Hoos A. (2015) Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov, 14 (9): 603-22. [PMID:26228631]
2. Ali AY, Wu X, Eissa N, Hou S, Ghia JE, Murooka TT, Banerji V, Johnston JB, Lin F, Gibson SB et al.. (2018) Distinct roles for phosphoinositide 3-kinases γ and δ in malignant B cell migration. Leukemia, 32 (9): 1958-1969. [PMID:29479062]
3. Allen RA, Brookings DC, Powell MJ, Delgado J, Shuttleworth LK, Merriman M, Fahy IJ, Tewari R, Silva JP, Healy LJ et al.. (2017) Seletalisib: Characterization of a Novel, Potent, and Selective Inhibitor of PI3Kδ. J Pharmacol Exp Ther, 361 (3): 429-440. [PMID:28442583]
4. Apsel B, Blair JA, Gonzalez B, Nazif TM, Feldman ME, Aizenstein B, Hoffman R, Williams RL, Shokat KM, Knight ZA. (2008) Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol, 4 (11): 691-9. [PMID:18849971]
5. Barda DA, Mader MM. (2013) PI3 kinase/mTOR dual inhibitor. Patent number: US8440829 B2. Assignee: Eli Lilly And Company. Priority date: 14/01/2011. Publication date: 14/05/2013.
6. Barlaam B, Cosulich S, Delouvrié B, Ellston R, Fitzek M, Germain H, Green S, Hancox U, Harris CS, Hudson K et al.. (2015) Discovery of 1-(4-(5-(5-amino-6-(5-tert-butyl-1,3,4-oxadiazol-2-yl)pyrazin-2-yl)-1-ethyl-1,2,4-triazol-3-yl)piperidin-1-yl)-3-hydroxypropan-1-one (AZD8835): A potent and selective inhibitor of PI3Kα and PI3Kδ for the treatment of cancers. Bioorg Med Chem Lett, 25 (22): 5155-62. [PMID:26475521]
7. Bergamini G, Bell K, Shimamura S, Werner T, Cansfield A, Müller K, Perrin J, Rau C, Ellard K, Hopf C et al.. (2012) A selective inhibitor reveals PI3Kγ dependence of T(H)17 cell differentiation. Nat Chem Biol, 8 (6): 576-82. [PMID:22544264]
8. Braun M-G, Hanan E, Staben ST, Heald RA, Macleod C, Elliott R. (2017) Benzoxazepin oxazolidinone compounds and methods of use. Patent number: US20170015678. Assignee: Genentech, Inc.. Priority date: 02/07/2015. Publication date: 19/01/2017.
9. Brown SD, Matthews DJ. (2012) (alpha- substituted aralkylamino and heteroarylalkylamino) pyrimidinyl and 1,3,5 -triazinyl benzimidazoles, pharmaceutical compositions containing them, and these compounds for use in treating proliferative diseases. Patent number: WO2012135160A1. Assignee: Pathway Therapeutics Inc.. Priority date: 28/03/2011. Publication date: 04/10/2012.
10. Camps M, Rückle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Françon B et al.. (2005) Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med, 11 (9): 936-43. [PMID:16127437]
11. Cano C, Saravanan K, Bailey C, Bardos J, Curtin NJ, Frigerio M, Golding BT, Hardcastle IR, Hummersone MG, Menear KA et al.. (2013) 1-substituted (Dibenzo[b,d]thiophen-4-yl)-2-morpholino-4H-chromen-4-ones endowed with dual DNA-PK/PI3-K inhibitory activity. J Med Chem, 56 (16): 6386-401. [PMID:23855836]
12. Castro-Falcón G, Seiler GS, Demir Ö, Rathinaswamy MK, Hamelin D, Hoffmann RM, Makowski SL, Letzel AC, Field SJ, Burke JE et al.. (2018) Neolymphostin A Is a Covalent Phosphoinositide 3-Kinase (PI3K)/Mammalian Target of Rapamycin (mTOR) Dual Inhibitor That Employs an Unusual Electrophilic Vinylogous Ester. J Med Chem, 61 (23): 10463-10472. [PMID:30380865]
13. Cheng H, Li C, Bailey S, Baxi SM, Goulet L, Guo L, Hoffman J, Jiang Y, Johnson TO, Johnson TW et al.. (2013) Discovery of the Highly Potent PI3K/mTOR Dual Inhibitor PF-04979064 through Structure-Based Drug Design. ACS Med Chem Lett, 4 (1): 91-7. [PMID:24900568]
14. Cherian PT, Koikov LN, Wortman MD, Knittel JJ. (2009) Exploring the PI3K alpha and gamma binding sites with 2,6-disubstituted isonicotinic derivatives. Bioorg Med Chem Lett, 19 (8): 2215-9. [PMID:19297156]
15. Collier PN, Martinez-Botella G, Cornebise M, Cottrell KM, Doran JD, Griffith JP, Mahajan S, Maltais F, Moody CS, Huck EP et al.. (2015) Structural basis for isoform selectivity in a class of benzothiazole inhibitors of phosphoinositide 3-kinase γ. J Med Chem, 58 (1): 517-21. [PMID:24754609]
16. Cushing TD, Hao X, Shin Y, Andrews K, Brown M, Cardozo M, Chen Y, Duquette J, Fisher B, Gonzalez-Lopez de Turiso F et al.. (2015) Discovery and in vivo evaluation of (S)-N-(1-(7-fluoro-2-(pyridin-2-yl)quinolin-3-yl)ethyl)-9H-purin-6-amine (AMG319) and related PI3Kδ inhibitors for inflammation and autoimmune disease. J Med Chem, 58 (1): 480-511. [PMID:25469863]
17. D'Angelo ND, Kim TS, Andrews K, Booker SK, Caenepeel S, Chen K, D'Amico D, Freeman D, Jiang J, Liu L et al.. (2011) Discovery and optimization of a series of benzothiazole phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors. J Med Chem, 54 (6): 1789-811. [PMID:21332118]
18. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
19. Down K, Amour A, Baldwin IR, Cooper AW, Deakin AM, Felton LM, Guntrip SB, Hardy C, Harrison ZA, Jones KL et al.. (2015) Optimization of Novel Indazoles as Highly Potent and Selective Inhibitors of Phosphoinositide 3-Kinase δ for the Treatment of Respiratory Disease. J Med Chem, 58 (18): 7381-99. [PMID:26301626]
20. Evans CA, Liu T, Lescarbeau A, Nair SJ, Grenier L, Pradeilles JA, Glenadel Q, Tibbitts T, Rowley AM, DiNitto JP et al.. (2016) Discovery of a Selective Phosphoinositide-3-Kinase (PI3K)-γ Inhibitor (IPI-549) as an Immuno-Oncology Clinical Candidate. ACS Med Chem Lett, 7 (9): 862-7. [PMID:27660692]
21. Fairhurst RA, Furet P, Imbach-Weese P, Stauffer F, Rueeger H, McCarthy C, Ripoche S, Oswald S, Arnaud B, Jary A et al.. (2022) Identification of NVP-CLR457 as an Orally Bioavailable Non-CNS-Penetrant pan-Class IA Phosphoinositol-3-Kinase Inhibitor. J Med Chem, 65 (12): 8345-8379. [PMID:35500094]
22. Fokas E, Prevo R, Pollard JR, Reaper PM, Charlton PA, Cornelissen B, Vallis KA, Hammond EM, Olcina MM, Gillies McKenna W et al.. (2012) Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis, 3: e441. [PMID:23222511]
23. Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, Chuckowree IS, Clarke PA, Depledge P, Eccles SA et al.. (2008) The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer . J Med Chem, 51 (18): 5522-32. [PMID:18754654]
24. Fraser C, Carragher NO, Unciti-Broceta A. (2016) eCF309: a potent, selective and cell-permeable mTOR inhibitor. Medchemcomm, 7 (3): 471-477.
25. Furet P, Guagnano V, Fairhurst RA, Imbach-Weese P, Bruce I, Knapp M, Fritsch C, Blasco F, Blanz J, Aichholz R et al.. (2013) Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg Med Chem Lett, 23 (13): 3741-8. [PMID:23726034]
26. Gangadhara G, Dahl G, Bohnacker T, Rae R, Gunnarsson J, Blaho S, Öster L, Lindmark H, Karabelas K, Pemberton N et al.. (2019) A class of highly selective inhibitors bind to an active state of PI3Kγ. Nat Chem Biol, 15 (4): 348-357. [PMID:30718815]
27. Goldberg FW, Finlay MRV, Ting AKT, Beattie D, Lamont GM, Fallan C, Wrigley GL, Schimpl M, Howard MR, Williamson B et al.. (2020) The Discovery of 7-Methyl-2-[(7-methyl[1,2,4]triazolo[1,5-a]pyridin-6-yl)amino]-9-(tetrahydro-2H-pyran-4-yl)-7,9-dihydro-8H-purin-8-one (AZD7648), a Potent and Selective DNA-Dependent Protein Kinase (DNA-PK) Inhibitor. J Med Chem, 63 (7): 3461-3471. [PMID:31851518]
28. Gopalsamy A, Bennett EM, Shi M, Zhang WG, Bard J, Yu K. (2012) Identification of pyrimidine derivatives as hSMG-1 inhibitors. Bioorg Med Chem Lett, 22 (21): 6636-41. [PMID:23021994]
29. Hancox U, Cosulich S, Hanson L, Trigwell C, Lenaghan C, Ellston R, Dry H, Crafter C, Barlaam B, Fitzek M et al.. (2015) Inhibition of PI3Kβ signaling with AZD8186 inhibits growth of PTEN-deficient breast and prostate tumors alone and in combination with docetaxel. Mol Cancer Ther, 14 (1): 48-58. [PMID:25398829]
30. Hart S, Novotny-Diermayr V, Goh KC, Williams M, Tan YC, Ong LC, Cheong A, Ng BK, Amalini C, Madan B et al.. (2013) VS-5584, a novel and highly selective PI3K/mTOR kinase inhibitor for the treatment of cancer. Mol Cancer Ther, 12 (2): 151-61. [PMID:23270925]
31. Hayakawa M, Kawaguchi K, Kaizawa H, Koizumi T, Ohishi T, Yamano M, Okada M, Ohta M, Tsukamoto S, Raynaud FI et al.. (2007) Synthesis and biological evaluation of sulfonylhydrazone-substituted imidazo[1,2-a]pyridines as novel PI3 kinase p110alpha inhibitors. Bioorg Med Chem, 15 (17): 5837-44. [PMID:17601739]
32. Henley ZA, Amour A, Barton N, Bantscheff M, Bergamini G, Bertrand SM, Convery M, Down K, Dümpelfeld B, Edwards CD et al.. (2020) Optimization of Orally Bioavailable PI3Kδ Inhibitors and Identification of Vps34 as a Key Selectivity Target. J Med Chem, 63 (2): 638-655. DOI: 10.1021/acs.jmedchem.9b01585 [PMID:31855425]
33. Hou Y, Zhang F, Min W, Yuan K, Kuang W, Wang X, Zhu Y, Sun C, Xia F, Wang Y et al.. (2022) Discovery of Novel Phosphoinositide-3-Kinase α Inhibitors with High Selectivity, Excellent Bioavailability, and Long-Acting Efficacy for Gastric Cancer. J Med Chem, 65 (14): 9873-9892. [PMID:35834807]
34. Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ et al.. (2012) The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature, 485 (7396): 55-61. [PMID:22367541]
35. Jalota-Badhwar A, Bhatia DR, Boreddy S, Joshi A, Venkatraman M, Desai N, Chaudhari S, Bose J, Kolla LS, Deore V et al.. (2015) P7170: A Novel Molecule with Unique Profile of mTORC1/C2 and Activin Receptor-like Kinase 1 Inhibition Leading to Antitumor and Antiangiogenic Activity. Mol Cancer Ther, 14 (5): 1095-106. [PMID:25700704]
36. Kashiyama T, Oda K, Ikeda Y, Shiose Y, Hirota Y, Inaba K, Makii C, Kurikawa R, Miyasaka A, Koso T et al.. (2014) Antitumor activity and induction of TP53-dependent apoptosis toward ovarian clear cell adenocarcinoma by the dual PI3K/mTOR inhibitor DS-7423. PLoS One, 9 (2): e87220. [PMID:24504419]
37. King-Underwood J, Ito K, Murray PJ, Brookfield FA, Brown CJ. (2012) QUINAZOLIN-4 (3H) -ONE DERIVATIVES USED AS PI3 KINASE INHIBITORS. Patent number: WO2012052753. Assignee: RESPIVERT LIMITED. Priority date: 18/10/2010. Publication date: 26/04/2012.
38. Knight SD, Adams ND, Burgess JL, Chaudhari AM, Darcy MG, Donatelli CA, Luengo JI, Newlander KA, Parrish CA, Ridgers LH et al.. (2010) Discovery of GSK2126458, a Highly Potent Inhibitor of PI3K and the Mammalian Target of Rapamycin. ACS Med Chem Lett, 1 (1): 39-43. [PMID:24900173]
39. Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B et al.. (2006) A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell, 125 (4): 733-47. [PMID:16647110]
40. Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M et al.. (2011) CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood, 117 (2): 591-4. [PMID:20959606]
41. Li Y-L, Metcalf BW, Combs AP. (2011) Pyrimidinones as PI3K inhibitors. Patent number: WO2011008487. Assignee: Incyte Corporation. Priority date: 29/06/2009. Publication date: 20/01/2011.
42. Lin S, Jin J, Liu Y, Tian H, Zhang Y, Fu R, Zhang J, Wang M, Du T, Ji M et al.. (2019) Discovery of 4-Methylquinazoline Based PI3K Inhibitors for the Potential Treatment of Idiopathic Pulmonary Fibrosis. J Med Chem, 62 (19): 8873-8879. [PMID:31335136]
43. Liu F, Wang J, Yang X, Li B, Wu H, Qi S, Chen C, Liu X, Yu K, Wang W et al.. (2016) Discovery of a Highly Selective STK16 Kinase Inhibitor. ACS Chem Biol, 11 (6): 1537-43. [PMID:27082499]
44. Liu N, Rowley BR, Bull CO, Schneider C, Haegebarth A, Schatz CA, Fracasso PR, Wilkie DP, Hentemann M, Wilhelm SM et al.. (2013) BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol Cancer Ther, 12 (11): 2319-30. [PMID:24170767]
45. Liu Q, Shi Q, Marcoux D, Batt DG, Cornelius L, Qin LY, Ruan Z, Neels J, Beaudoin-Bertrand M, Srivastava AS et al.. (2017) Identification of a Potent, Selective, and Efficacious Phosphatidylinositol 3-Kinase δ (PI3Kδ) Inhibitor for the Treatment of Immunological Disorders. J Med Chem, 60 (12): 5193-5208. [PMID:28541707]
46. Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker K et al.. (2008) Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther, 7 (7): 1851-63. [PMID:18606717]
47. Markman B, Tabernero J, Krop I, Shapiro GI, Siu L, Chen LC, Mita M, Melendez Cuero M, Stutvoet S, Birle D et al.. (2012) Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann Oncol, 23 (9): 2399-408. [PMID:22357447]
48. Methot JL, Zhou H, Kattar SD, McGowan MA, Wilson K, Garcia Y, Deng Y, Altman M, Fradera X, Lesburg C et al.. (2019) Structure Overhaul Affords a Potent Purine PI3Kδ Inhibitor with Improved Tolerability. J Med Chem, 62 (9): 4370-4382. [PMID:30986068]
49. Mårdh CK, Root J, Uddin M, Stenvall K, Malmgren A, Karabelas K, Thomas M. (2017) Targets of Neutrophil Influx and Weaponry: Therapeutic Opportunities for Chronic Obstructive Airway Disease. J Immunol Res, 2017: 5273201. [PMID:28596972]
50. Ndubaku CO, Heffron TP, Staben ST, Baumgardner M, Blaquiere N, Bradley E, Bull R, Do S, Dotson J, Dudley D et al.. (2013) Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a β-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J Med Chem, 56 (11): 4597-610. [PMID:23662903]
51. Nylander S, Kull B, Björkman JA, Ulvinge JC, Oakes N, Emanuelsson BM, Andersson M, Skärby T, Inghardt T, Fjellström O et al.. (2012) Human target validation of phosphoinositide 3-kinase (PI3K)β: effects on platelets and insulin sensitivity, using AZD6482 a novel PI3Kβ inhibitor. J Thromb Haemost, 10 (10): 2127-36. [PMID:22906130]
52. Ohwada J, Ebiike H, Kawada H, Tsukazaki M, Nakamura M, Miyazaki T, Morikami K, Yoshinari K, Yoshida M, Kondoh O et al.. (2011) Discovery and biological activity of a novel class I PI3K inhibitor, CH5132799. Bioorg Med Chem Lett, 21 (6): 1767-72. [PMID:21316229]
53. Palanki MS, Dneprovskaia E, Doukas J, Fine RM, Hood J, Kang X, Lohse D, Martin M, Noronha G, Soll RM et al.. (2007) Discovery of 3,3'-(2,4-diaminopteridine-6,7-diyl)diphenol as an isozyme-selective inhibitor of PI3K for the treatment of ischemia reperfusion injury associated with myocardial infarction. J Med Chem, 50 (18): 4279-94. [PMID:17685602]
54. Pemberton N, Mogemark M, Arlbrandt S, Bold P, Cox RJ, Gardelli C, Holden NS, Karabelas K, Karlsson J, Lever S et al.. (2018) Discovery of Highly Isoform Selective Orally Bioavailable Phosphoinositide 3-Kinase (PI3K)-γ Inhibitors. J Med Chem, 61 (12): 5435-5441. [PMID:29852070]
55. Perry M, Karabelas K, Mogemark M, Bold P, Tyrchan C, Nikitidid A, Petersen J, Borjesson U. (2018) 5-[2-(pyridin-2-ylamino)-1,3-thiazol-5-yl]-2,3-dihydro-1 h-isoindol-1 -one derivatives and their use as dual inhibitors of phosphatidylinositol 3-kinase delta & gamma. Patent number: WO2018055040A1. Assignee: Astrazeneca Ab. Priority date: 22/09/2016. Publication date: 29/03/2018.
56. Pomel V, Klicic J, Covini D, Church DD, Shaw JP, Roulin K, Burgat-Charvillon F, Valognes D, Camps M, Chabert C et al.. (2006) Furan-2-ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3-kinase gamma. J Med Chem, 49 (13): 3857-71. [PMID:16789742]
57. Qian C, Lai CJ, Bao R, Wang DG, Wang J, Xu GX, Atoyan R, Qu H, Yin L, Samson M et al.. (2012) Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling. Clin Cancer Res, 18 (15): 4104-13. [PMID:22693356]
58. Raynaud FI, Eccles SA, Patel S, Alix S, Box G, Chuckowree I, Folkes A, Gowan S, De Haven Brandon A, Di Stefano F et al.. (2009) Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther, 8 (7): 1725-38. [PMID:19584227]
59. Ren P, Liu Y, Li L, Chan K, Wilson TE, Campbell SF. (2013) Heterocyclic compounds and uses thereof. Patent number: US20130035324 A1. Assignee: Ren P, Liu Y, Li L, Chan K, Wilson TE, Campbell SF.. Priority date: 17/08/2009. Publication date: 07/02/2013.
60. Shugg RP, Thomson A, Tanabe N, Kashishian A, Steiner BH, Puri KD, Pereverzev A, Lannutti BJ, Jirik FR, Dixon SJ et al.. (2013) Effects of isoform-selective phosphatidylinositol 3-kinase inhibitors on osteoclasts: actions on cytoskeletal organization, survival, and resorption. J Biol Chem, 288 (49): 35346-57. [PMID:24133210]
61. Su W-G, Dai G, Zhang W, Deng W. (2016) Novel imidazopyridazine compounds and their use. Patent number: WO2016045591A1. Assignee: Hutchison Medipharma Limited. Priority date: 24/09/2014. Publication date: 31/03/2016.
62. Sutherlin DP, Bao L, Berry M, Castanedo G, Chuckowree I, Dotson J, Folks A, Friedman L, Goldsmith R, Gunzner J et al.. (2011) Discovery of a potent, selective, and orally available class I phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) kinase inhibitor (GDC-0980) for the treatment of cancer. J Med Chem, 54 (21): 7579-87. [PMID:21981714]
63. Taddei DMA, Onions ST, Smith AJ, Copmans AH, Broeckx RLM. (2016) Phosphoinositide 3-kinase inhibitors. Patent number: US9227977B2. Assignee: Respivert Ltd. Priority date: 15/03/2013. Publication date: 05/01/2016.
64. Uddin M, Lau LC, Seumois G, Vijayanand P, Staples KJ, Bagmane D, Cornelius V, Dorinsky P, Davies DE, Djukanović R. (2013) EGF-induced bronchial epithelial cells drive neutrophil chemotactic and anti-apoptotic activity in asthma. PLoS ONE, 8 (9): e72502. [PMID:24039773]
65. Vakkalanka SKVS, Bhavar PK, Viswanadha S, Babu G. (2017) Dual selective PI3 delta and gamma kinase inhibitors. Patent number: US9790224B2. Assignee: Rhizen Pharmaceuticals SA. Priority date: 07/06/2013. Publication date: 17/10/2017.
66. Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. (2000) Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell, 6 (4): 909-19. [PMID:11090628]
67. Winkler DG, Faia KL, DiNitto JP, Ali JA, White KF, Brophy EE, Pink MM, Proctor JL, Lussier J, Martin CM et al.. (2013) PI3K-δ and PI3K-γ inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol, 20 (11): 1364-74. [PMID:24211136]
68. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
69. Wu P, Hu Y. (2012) Small molecules targeting phosphoinositide 3-kinases. Medchemcomm, 3 (11): 1337-1355. DOI: 10.1039/C2MD20044A
70. Xie C, He Y, Zhen M, Wang Y, Xu Y, Lou L. (2017) Puquitinib, a novel orally available PI3Kδ inhibitor, exhibits potent antitumor efficacy against acute myeloid leukemia. Cancer Sci, 108 (7): 1476-1484. [PMID:28418085]
71. Yaguchi S, Fukui Y, Koshimizu I, Yoshimi H, Matsuno T, Gouda H, Hirono S, Yamazaki K, Yamori T. (2006) Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J Natl Cancer Inst, 98 (8): 545-56. [PMID:16622124]
72. Yang J, Shamji A, Matchacheep S, Schreiber SL. (2007) Identification of a small-molecule inhibitor of class Ia PI3Ks with cell-based screening. Chem Biol, 14 (4): 371-7. [PMID:17462572]
73. Yu Y, Han Y, Zhang F, Gao Z, Zhu T, Dong S, Ma M. (2020) Design, Synthesis, and Biological Evaluation of Imidazo[1,2-a]pyridine Derivatives as Novel PI3K/mTOR Dual Inhibitors. J Med Chem, 63 (6): 3028-3046. [PMID:32069401]
74. Zhan X, Su L. (2021) Three fused ring derivative-containing salt or crystal form and pharmaceutical composition thereof. Patent number: WO2021104146A1. Assignee: Shanghai Hansen Biomedical Technology, Jiangsu Hansen Pharmaceutical Group. Priority date: 25/11/2019. Publication date: 03/06/2021.