1. Al-Ghoul WM, Herman MD, Dubocovich ML. (1998) Melatonin receptor subtype expression in human cerebellum.
Neuroreport, 9 (18): 4063-8.
[PMID:9926848]
2. Andersson EA, Holst B, Sparsø T, Grarup N, Banasik K, Holmkvist J, Jørgensen T, Borch-Johnsen K, Egerod KL, Lauritzen T et al.. (2010) MTNR1B G24E variant associates With BMI and fasting plasma glucose in the general population in studies of 22,142 Europeans.
Diabetes, 59 (6): 1539-48.
[PMID:20200315]
3. Audinot V, Bonnaud A, Grandcolas L, Rodriguez M, Nagel N, Galizzi JP, Balik A, Messager S, Hazlerigg DG, Barrett P et al.. (2008) Molecular cloning and pharmacological characterization of rat melatonin MT1 and MT2 receptors.
Biochem Pharmacol, 75 (10): 2007-19.
[PMID:18384758]
4. Audinot V, Mailliet F, Lahaye-Brasseur C, Bonnaud A, Le Gall A, Amossé C, Dromaint S, Rodriguez M, Nagel N, Galizzi JP et al.. (2003) New selective ligands of human cloned melatonin MT1 and MT2 receptors.
Naunyn Schmiedebergs Arch Pharmacol, 367 (6): 553-61.
[PMID:12764576]
5. Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, Jockers R. (2002) Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer.
J Biol Chem, 277 (24): 21522-8.
[PMID:11940583]
6. Ayoub MA, Levoye A, Delagrange P, Jockers R. (2004) Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers.
Mol Pharmacol, 66 (2): 312-21.
[PMID:15266022]
7. Baba K, Benleulmi-Chaachoua A, Journé AS, Kamal M, Guillaume JL, Dussaud S, Gbahou F, Yettou K, Liu C, Contreras-Alcantara S et al.. (2013) Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function.
Sci Signal, 6 (296): ra89.
[PMID:24106342]
8. Benleulmi-Chaachoua A, Hegron A, Le Boulch M, Karamitri A, Wierzbicka M, Wong V, Stagljar I, Delagrange P, Ahmad R, Jockers R. (2018) Melatonin receptors limit dopamine reuptake by regulating dopamine transporter cell-surface exposure.
Cell Mol Life Sci, 75 (23): 4357-4370.
[PMID:30043140]
9. Beresford IJ, Browning C, Starkey SJ, Brown J, Foord SM, Coughlan J, North PC, Dubocovich ML, Hagan RM. (1998) GR196429: a nonindolic agonist at high-affinity melatonin receptors.
J Pharmacol Exp Ther, 285 (3): 1239-45.
[PMID:9618428]
10. Bonnefond A, Clément N, Fawcett K, Yengo L, Vaillant E, Guillaume JL, Dechaume A, Payne F, Roussel R, Czernichow S et al.. (2012) Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes.
Nat Genet, 44 (3): 297-301.
[PMID:22286214]
11. Browning C, Beresford I, Fraser N, Giles H. (2000) Pharmacological characterization of human recombinant melatonin mt(1) and MT(2) receptors.
Br J Pharmacol, 129 (5): 877-86.
[PMID:10696085]
12. Brydon L, Petit L, Delagrange P, Strosberg AD, Jockers R. (2001) Functional expression of MT2 (Mel1b) melatonin receptors in human PAZ6 adipocytes.
Endocrinology, 142 (10): 4264-71.
[PMID:11564683]
13. Chaste P, Clement N, Mercati O, Guillaume JL, Delorme R, Botros HG, Pagan C, Périvier S, Scheid I, Nygren G, Anckarsäter H, Rastam M, Ståhlberg O, Gillberg C, Serrano E, Lemière N, Launay JM, Mouren-Simeoni MC, Leboyer M, Gillberg C, Jockers R, Bourgeron T. (2010) Identification of pathway-biased and deleterious melatonin receptor mutants in autism spectrum disorders and in the general population.
PLoS ONE, 5 (7): e11495.
[PMID:20657642]
14. Cogé F, Guenin SP, Fery I, Migaud M, Devavry S, Slugocki C, Legros C, Ouvry C, Cohen W, Renault N et al.. (2009) The end of a myth: cloning and characterization of the ovine melatonin MT(2) receptor.
Br J Pharmacol, 158 (5): 1248-62.
[PMID:19814723]
15. Contreras-Alcantara S, Baba K, Tosini G. (2010) Removal of melatonin receptor type 1 induces insulin resistance in the mouse.
Obesity (Silver Spring), 18 (9): 1861-3.
[PMID:20168308]
16. Doolen S, Krause DN, Dubocovich ML, Duckles SP. (1998) Melatonin mediates two distinct responses in vascular smooth muscle.
Eur J Pharmacol, 345 (1): 67-9.
[PMID:9593596]
17. Drazen DL, Bilu D, Bilbo SD, Nelson RJ. (2001) Melatonin enhancement of splenocyte proliferation is attenuated by luzindole, a melatonin receptor antagonist.
Am J Physiol Regul Integr Comp Physiol, 280 (5): R1476-82.
[PMID:11294771]
18. Drazen DL, Nelson RJ. (2001) Melatonin receptor subtype MT2 (Mel 1b) and not mt1 (Mel 1a) is associated with melatonin-induced enhancement of cell-mediated and humoral immunity.
Neuroendocrinology, 74 (3): 178-84.
[PMID:11528219]
19. Drew JE, Williams LM, Hannah LT, Barrett P, Abramovich DR. (1998) Melatonin receptors in the human fetal kidney: 2-[125I]iodomelatonin binding sites correlated with expression of Mel1a and Mel1b receptor genes.
J Endocrinol, 156 (2): 261-7.
[PMID:9518871]
20. Dubocovich ML. (1985) Characterization of a retinal melatonin receptor.
J Pharmacol Exp Ther, 234 (2): 395-401.
[PMID:2991499]
21. Dubocovich ML. (1988) Luzindole (N-0774): a novel melatonin receptor antagonist.
J Pharmacol Exp Ther, 246 (3): 902-10.
[PMID:2843633]
22. Dubocovich ML, Hudson RL, Sumaya IC, Masana MI, Manna E. (2005) Effect of MT1 melatonin receptor deletion on melatonin-mediated phase shift of circadian rhythms in the C57BL/6 mouse.
J Pineal Res, 39 (2): 113-20.
[PMID:16098087]
23. Dubocovich ML, Masana MI, Iacob S, Sauri DM. (1997) Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor.
Naunyn Schmiedebergs Arch Pharmacol, 355 (3): 365-75.
[PMID:9089668]
24. Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI. (2003) Molecular pharmacology, regulation and function of mammalian melatonin receptors.
Front Biosci, 8: d1093-108.
[PMID:12957828]
25. Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI. (1998) Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms.
FASEB J, 12 (12): 1211-20.
[PMID:9737724]
26. Ebisawa T, Uchiyama M, Kajimura N, Kamei Y, Shibui K, Kim K, Kudo Y, Iwase T, Sugishita M, Jodoi T et al.. (2000) Genetic polymorphisms of human melatonin 1b receptor gene in circadian rhythm sleep disorders and controls.
Neurosci Lett, 280 (1): 29-32.
[PMID:10696804]
27. Ettaoussi M, Sabaouni A, Rami M, Boutin JA, Delagrange P, Renard P, Spedding M, Caignard DH, Berthelot P, Yous S. (2012) Design, synthesis and pharmacological evaluation of new series of naphthalenic analogues as melatoninergic (MT1/MT2) and serotoninergic 5-HT2C dual ligands (I).
Eur J Med Chem, 49: 310-23.
[PMID:22301214]
28. Faust R, Garratt PJ, Jones R, Yeh LK, Tsotinis A, Panoussopoulou M, Calogeropoulou T, Teh MT, Sugden D. (2000) Mapping the melatonin receptor. 6. Melatonin agonists and antagonists derived from 6H-isoindolo[2,1-a]indoles, 5,6-dihydroindolo[2,1-a]isoquinolines, and 6,7-dihydro-5H-benzo[c]azepino[2,1-a]indoles.
J Med Chem, 43 (6): 1050-61.
[PMID:10737738]
29. Gbahou F, Cecon E, Viault G, Gerbier R, Jean-Alphonse F, Karamitri A, Guillaumet G, Delagrange P, Friedlander RM, Vilardaga JP et al.. (2017) Design and validation of the first cell-impermeant melatonin receptor agonist.
Br J Pharmacol, 174 (14): 2409-2421.
[PMID:28493341]
30. Hu Y, Zhu J, Chan KH, Wong YH. (2013) Development of substituted N-[3-(3-methoxylphenyl)propyl] amides as MT(2)-selective melatonin agonists: improving metabolic stability.
Bioorg Med Chem, 21 (2): 547-52.
[PMID:23228808]
31. Hunt AE, Al-Ghoul WM, Gillette MU, Dubocovich ML. (2001) Activation of MT(2) melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock.
Am J Physiol, Cell Physiol, 280 (1): C110-8.
[PMID:11121382]
32. Jin X, von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, Weaver DR. (2003) Targeted disruption of the mouse Mel(1b) melatonin receptor.
Mol Cell Biol, 23 (3): 1054-60.
[PMID:12529409]
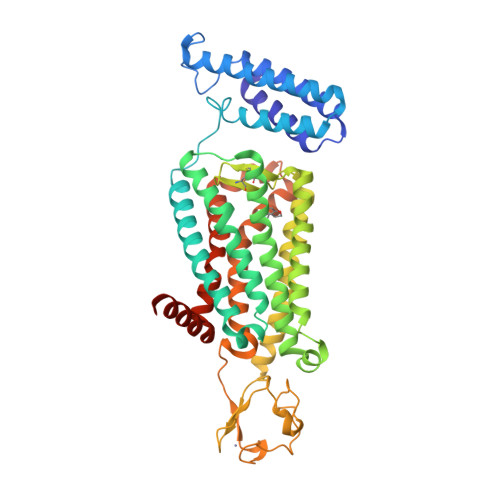
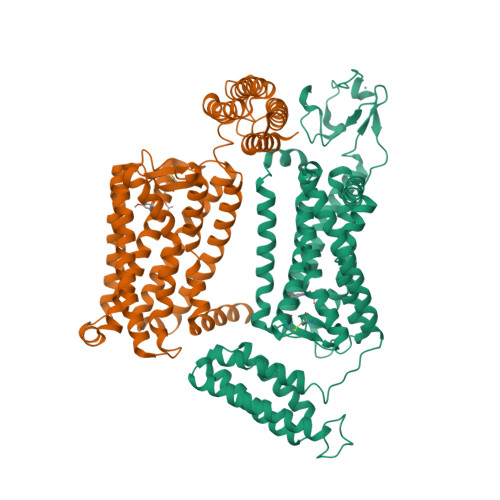
33. Johansson LC, Stauch B, McCorvy JD, Han GW, Patel N, Huang XP, Batyuk A, Gati C, Slocum ST, Li C et al.. (2019) XFEL structures of the human MT2 melatonin receptor reveal the basis of subtype selectivity.
Nature, 569 (7755): 289-292.
[PMID:31019305]
34. Kato K, Hirai K, Nishiyama K, Uchikawa O, Fukatsu K, Ohkawa S, Kawamata Y, Hinuma S, Miyamoto M. (2005) Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist.
Neuropharmacology, 48 (2): 301-10.
[PMID:15695169]
35. Koike T, Hoashi Y, Takai T, Nakayama M, Yukuhiro N, Ishikawa T, Hirai K, Uchikawa O. (2011) 1,6-Dihydro-2H-indeno[5,4-b]furan derivatives: design, synthesis, and pharmacological characterization of a novel class of highly potent MT₂-selective agonists.
J Med Chem, 54 (9): 3436-44.
[PMID:21473625]
36. Koike T, Takai T, Hoashi Y, Nakayama M, Kosugi Y, Nakashima M, Yoshikubo S, Hirai K, Uchikawa O. (2011) Synthesis of a novel series of tricyclic dihydrofuran derivatives: discovery of 8,9-dihydrofuro[3,2-c]pyrazolo[1,5-a]pyridines as melatonin receptor (MT1/MT2) ligands.
J Med Chem, 54 (12): 4207-18.
[PMID:21568291]
37. Lanoix D, Ouellette R, Vaillancourt C. (2006) Expression of melatoninergic receptors in human placental choriocarcinoma cell lines.
Hum Reprod, 21 (8): 1981-9.
[PMID:16632463]
38. Legros C, Brasseur C, Delagrange P, Ducrot P, Nosjean O, Boutin JA. (2016) Alternative Radioligands for Investigating the Molecular Pharmacology of Melatonin Receptors.
J Pharmacol Exp Ther, 356 (3): 681-92.
[PMID:26759496]
39. Legros C, Matthey U, Grelak T, Pedragona-Moreau S, Hassler W, Yous S, Thomas E, Suzenet F, Folleas B, Lefoulon F et al.. (2013) New Radioligands for Describing the Molecular Pharmacology of MT1 and MT2 Melatonin Receptors.
Int J Mol Sci, 14 (5): 8948-62.
[PMID:23698757]
40. Levoye A, Dam J, Ayoub MA, Guillaume JL, Couturier C, Delagrange P, Jockers R. (2006) The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization.
EMBO J, 25 (13): 3012-23.
[PMID:16778767]
41. Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. (1997) Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock.
Neuron, 19 (1): 91-102.
[PMID:9247266]
42. Lucini V, Pannacci M, Scaglione F, Fraschini F, Rivara S, Mor M, Bordi F, Plazzi PV, Spadoni G, Bedini A et al.. (2004) Tricyclic alkylamides as melatonin receptor ligands with antagonist or inverse agonist activity.
J Med Chem, 47 (17): 4202-12.
[PMID:15293992]
43. MacKenzie RS, Melan MA, Passey DK, Witt-Enderby PA. (2002) Dual coupling of MT(1) and MT(2) melatonin receptors to cyclic AMP and phosphoinositide signal transduction cascades and their regulation following melatonin exposure.
Biochem Pharmacol, 63 (4): 587-95.
[PMID:11992626]
44. Masana MI, Doolen S, Ersahin C, Al-Ghoul WM, Duckles SP, Dubocovich ML, Krause DN. (2002) MT(2) melatonin receptors are present and functional in rat caudal artery.
J Pharmacol Exp Ther, 302: 1295-1302.
[PMID:12183692]
45. Masana MI, Dubocovich ML. (2001) Melatonin receptor signaling: finding the path through the dark.
Sci STKE, 2001 (107): pe39.
[PMID:11698691]
46. Mulchahey JJ, Goldwater DR, Zemlan FP. (2004) A single blind, placebo controlled, across groups dose escalation study of the safety, tolerability, pharmacokinetics and pharmacodynamics of the melatonin analog beta-methyl-6-chloromelatonin.
Life Sci, 75 (15): 1843-56.
[PMID:15302228]
47. Naji L, Carrillo-Vico A, Guerrero JM, Calvo JR. (2004) Expression of membrane and nuclear melatonin receptors in mouse peripheral organs.
Life Sci, 74: 2227-2236.
[PMID:14987948]
48. Niles LP, Wang J, Shen L, Lobb DK, Younglai EV. (1999) Melatonin receptor mRNA expression in human granulosa cells.
Mol Cell Endocrinol, 156 (1-2): 107-10.
[PMID:10612428]
49. Nonno R, Lucini V, Spadoni G, Pannacci M, Croce A, Esposti D, Balsamini C, Tarzia G, Fraschini F, Stankov BM. (2000) A new melatonin receptor ligand with mt1-agonist and MT2-antagonist properties.
J Pineal Res, 29 (4): 234-40.
[PMID:11068946]
50. Petit L, Lacroix I, de Coppet P, Strosberg AD, Jockers R. (1999) Differential signaling of human Mel1a and Mel1b melatonin receptors through the cyclic guanosine 3'-5'-monophosphate pathway.
Biochem Pharmacol, 58 (4): 633-9.
[PMID:10413300]
51. Poissonnier-Durieux S, Ettaoussi M, Pérès B, Boutin JA, Audinot V, Bennejean C, Delagrange P, Caignard DH, Renard P, Berthelot P et al.. (2008) Synthesis of 3-phenylnaphthalenic derivatives as new selective MT(2) melatoninergic ligands.
Bioorg Med Chem, 16 (18): 8339-48.
[PMID:18778943]
52. Rajaratnam SM, Polymeropoulos MH, Fisher DM, Roth T, Scott C, Birznieks G, Klerman EB. (2009) Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials.
Lancet, 373 (9662): 482-91.
[PMID:19054552]
53. Rawashdeh O, Hudson RL, Stepien I, Dubocovich ML. (2011) Circadian periods of sensitivity for ramelteon on the onset of running-wheel activity and the peak of suprachiasmatic nucleus neuronal firing rhythms in C3H/HeN mice.
Chronobiol Int, 28 (1): 31-8.
[PMID:21182402]
54. Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. (1995) Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor.
Proc Natl Acad Sci USA, 92 (19): 8734-8.
[PMID:7568007]
55. Reppert SM, Weaver DR, Godson C. (1996) Melatonin receptors step into the light: cloning and classification of subtypes.
Trends Pharmacol Sci, 17 (3): 100-2.
[PMID:8936344]
56. Rivara S, Lodola A, Mor M, Bedini A, Spadoni G, Lucini V, Pannacci M, Fraschini F, Scaglione F, Sanchez RO et al.. (2007) N-(substituted-anilinoethyl)amides: design, synthesis, and pharmacological characterization of a new class of melatonin receptor ligands.
J Med Chem, 50 (26): 6618-26.
[PMID:18052314]
57. Rivara S, Lorenzi S, Mor M, Plazzi PV, Spadoni G, Bedini A, Tarzia G. (2005) Analysis of structure-activity relationships for MT2 selective antagonists by melatonin MT1 and MT2 receptor models.
J Med Chem, 48 (12): 4049-60.
[PMID:15943478]
58. Roberts JE, Wiechmann AF, Hu DN. (2000) Melatonin receptors in human uveal melanocytes and melanoma cells.
J Pineal Res, 28 (3): 165-71.
[PMID:10739303]
59. Sallinen P, Saarela S, Ilves M, Vakkuri O, Leppäluoto J. (2005) The expression of MT1 and MT2 melatonin receptor mRNA in several rat tissues.
Life Sci, 76 (10): 1123-34.
[PMID:15620576]
60. Savaskan E, Ayoub MA, Ravid R, Angeloni D, Fraschini F, Meier F, Eckert A, Müller-Spahn F, Jockers R. (2005) Reduced hippocampal MT2 melatonin receptor expression in Alzheimer's disease.
J Pineal Res, 38 (1): 10-6.
[PMID:15617532]
61. Shiu SY, Li L, Xu JN, Pang CS, Wong JT, Pang SF. (1999) Melatonin-induced inhibition of proliferation and G1/S cell cycle transition delay of human choriocarcinoma JAr cells: possible involvement of MT2 (MEL1B) receptor.
J Pineal Res, 27 (3): 183-92.
[PMID:10535768]
62. Soares Jr JM, Masana MI, Erşahin C, Dubocovich ML. (2003) Functional melatonin receptors in rat ovaries at various stages of the estrous cycle.
J Pharmacol Exp Ther, 306 (2): 694-702.
[PMID:12721330]
63. Spadoni G, Bedini A, Diamantini G, Tarzia G, Rivara S, Lorenzi S, Lodola A, Mor M, Lucini V, Pannacci M et al.. (2007) Synthesis, enantiomeric resolution, and structure-activity relationship study of a series of 10,11-dihydro-5H-dibenzo[a,d]cycloheptene MT2 receptor antagonists.
ChemMedChem, 2 (12): 1741-9.
[PMID:17907131]
64. Spadoni G, Bedini A, Furiassi L, Mari M, Mor M, Scalvini L, Lodola A, Ghidini A, Lucini V, Dugnani S et al.. (2018) Identification of Bivalent Ligands with Melatonin Receptor Agonist and Fatty Acid Amide Hydrolase (FAAH) Inhibitory Activity That Exhibit Ocular Hypotensive Effect in the Rabbit.
J Med Chem, 61 (17): 7902-7916.
[PMID:30126274]
65. Spadoni G, Bedini A, Lucarini S, Mari M, Caignard DH, Boutin JA, Delagrange P, Lucini V, Scaglione F, Lodola A et al.. (2015) Highly Potent and Selective MT2 Melatonin Receptor Full Agonists from Conformational Analysis of 1-Benzyl-2-acylaminomethyl-tetrahydroquinolines.
J Med Chem, 58 (18): 7512-25.
[PMID:26334942]
66. Stein RM, Kang HJ, McCorvy JD, Glatfelter GC, Jones AJ, Che T, Slocum S, Huang XP, Savych O, Moroz YS et al.. (2020) Virtual discovery of melatonin receptor ligands to modulate circadian rhythms.
Nature, 579 (7800): 609-614.
[PMID:32040955]
67. Sugden D, Yeh LK, Teh MT. (1999) Design of subtype selective melatonin receptor agonists and antagonists.
Reprod Nutr Dev, 39 (3): 335-44.
[PMID:10420436]
68. Sumaya IC, Masana MI, Dubocovich ML. (2005) The antidepressant-like effect of the melatonin receptor ligand luzindole in mice during forced swimming requires expression of MT2 but not MT1 melatonin receptors.
J Pineal Res, 39 (2): 170-7.
[PMID:16098095]
69. Teh MT, Sugden D. (1998) Comparison of the structure-activity relationships of melatonin receptor agonists and antagonists: lengthening the N-acyl side-chain has differing effects on potency on Xenopus melanophores.
Naunyn Schmiedebergs Arch Pharmacol, 358 (5): 522-8.
[PMID:9840420]
70. Teh MT, Sugden D. (1999) The putative melatonin receptor antagonist GR128107 is a partial agonist on Xenopus laevis melanophores.
Br J Pharmacol, 126 (5): 1237-45.
[PMID:10205014]
71. Vanda Pharmaceuticals. Tasimelteon Advisory Committee Meeting Briefing Materials.
Accessed on 08/10/2014. Modified on 08/10/2014.
FDA, http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystemDrugsAdvisoryCommittee/UCM374388.pdf
72. Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS. (2005) Melatonin inhibits hippocampal long-term potentiation.
Eur J Neurosci, 22 (9): 2231-7.
[PMID:16262661]
73. Zlotos DP, Attia MI, Julius J, Sethi S, Witt-Enderby PA. (2009) 2-[(2,3-dihydro-1H-indol-1-yl)methyl]melatonin analogues: a novel class of MT2-selective melatonin receptor antagonists.
J Med Chem, 52 (3): 826-33.
[PMID:19193160]