
Top ▲

Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 425 | 1p35.2 | HCRTR1 | hypocretin receptor 1 | 84 |
| Mouse | 7 | 416 | 4 D2.2 | Hcrtr1 | hypocretin (orexin) receptor 1 | 17,84 |
| Rat | 7 | 416 | 5q36 | Hcrtr1 | hypocretin receptor 1 | 84 |
Previous and Unofficial Names  |
| Hctr1 | hypocretin receptor 1 | orexin receptor type 1 | OX1R |
Database Links  |
|
| Specialist databases | |
| GPCRdb | ox1r_human (Hs), ox1r_rat (Rn) |
| Other databases | |
| Alphafold | O43613 (Hs), P58307 (Mm), P56718 (Rn) |
| ChEMBL Target | CHEMBL5113 (Hs), CHEMBL2434819 (Mm), CHEMBL1075232 (Rn) |
| Ensembl Gene | ENSG00000121764 (Hs), ENSMUSG00000028778 (Mm), ENSRNOG00000013838 (Rn) |
| Entrez Gene | 3061 (Hs), 230777 (Mm), 25593 (Rn) |
| Human Protein Atlas | ENSG00000121764 (Hs) |
| KEGG Gene | hsa:3061 (Hs), mmu:230777 (Mm), rno:25593 (Rn) |
| OMIM | 602392 (Hs) |
| Pharos | O43613 (Hs) |
| RefSeq Nucleotide | NM_001525 (Hs), NM_198959 (Mm), NM_013064 (Rn) |
| RefSeq Protein | NP_001516 (Hs), NP_945197 (Mm), NP_037196 (Rn) |
| SynPHARM |
83546 (in complex with SB-674042) 6603 (in complex with suvorexant) |
| UniProtKB | O43613 (Hs), P58307 (Mm), P56718 (Rn) |
| Wikipedia | HCRTR1 (Hs) |
Selected 3D Structures  |
|||||||||||||
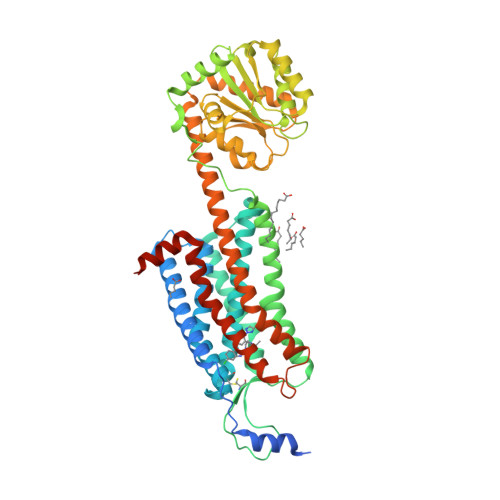
|
|
||||||||||||
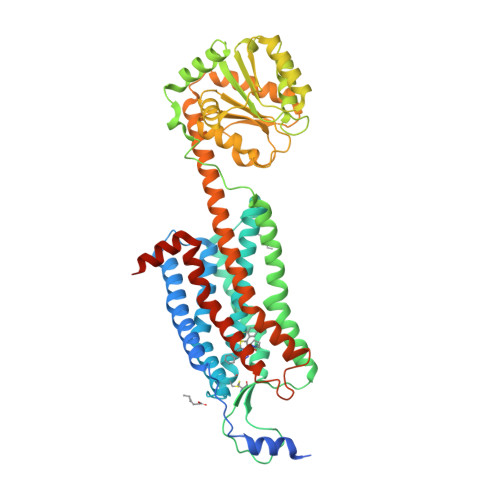
|
|
||||||||||||
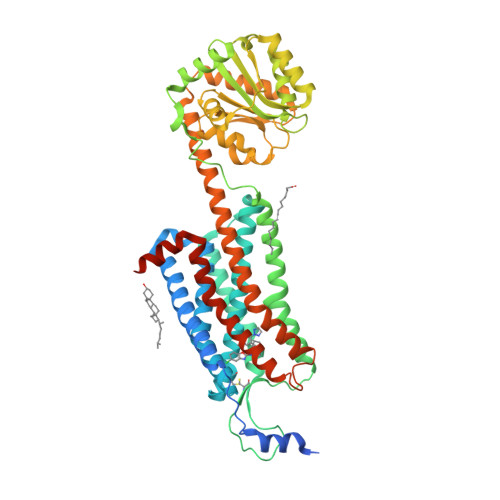
|
|
||||||||||||
Natural/Endogenous Ligands  |
| orexin-A {Sp: Human, Mouse, Rat} |
| orexin-B {Sp: Human} , orexin-B {Sp: Mouse, Rat} |
| Potency order of endogenous ligands |
| orexin-A (HCRT, O43612) > orexin-B (HCRT, O43612) (for Ca2+ elevation, unclear/variable for other responses) |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Efficacy values for agonists are highly dependent on cell type, assay conditions and the readout. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other Binding Ligands | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
| Gq/G11 family |
Adenylyl cyclase stimulation Adenylyl cyclase inhibition Phospholipase C stimulation Phospholipase A2 stimulation Phospholipase D stimulation |
| Comments: Association with the Gq family of transducers leads to phospholipase stimulation (e.g. phospholipase families A2, C and D) and Ca2+ elevation, and with the Gi family to inhibition and with the Gs family to stimulation, respectively, of adenylyl cyclase. Ca2+/non-selective cation influx also appears to rely on Gq. However, the signal transduction has not been investigated in detail in native neurons. | |
| References: 2,33,35,42,49-51,53,57-58,60,65,71,84,88,93-94,96 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
| Tissue Distribution Comments | ||||||||
| For IHC studies, it is very important to note that selectivity issues have been raised regarding antibodies for the orexin receptors which may lead to false positive/negative results and as such, mRNA expression patterns provide important confirmatory results. | ||||||||
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||
|
|||||||||||||
| General Comments |
|
No consequent differences in the molecular details of the signalling of the orexin receptor subtypes can be pointed out. In the CNS, both receptor subtypes regulate ion channel activity, most centrally leading to depolarization via activation of non-selective cation channels and inhibition of K+ channels [47,53,57]. Also, activation of Na+/Ca2+ exchanger has been reported. Orexin receptor activation has been implicated in modulation of synaptic plasticity. In native cells, cell lines and recombinant cells expressing heterologous orexin receptors, a wide set of molecular responses have been described. The immediate responses include activation of G proteins of Gq, Gi and Gs families and β-arrestin. Gq regulates at least receptor-operated Ca2+/non-selective cation influx and phospholipase C-mediated Ca2+ release, while Gi and Gs regulate adenylyl cyclase [48,50,53,57]. Orexin receptor subtypes form homo- and heteromeric complexes in recombinant systems. In addition, they are known to heteromerize with at least CB1 cannabinoid, κ opioid and CRF1 corticotropin receptors as well as with GPR103 [Reference: Kukkonen JP, Orexin/hypocretin Signaling, in Current Topics in Behavioral Neuroscience: Behavioral Neuroscience of Orexin/Hypocretin; ed. Andrew J Lawrence & Luis De Lecea, Springer; accepted for publication]. Little physiological significance has thus far been shown for this complex formation, except for a recent study showing complexes of OX1, CRF1 and σ-1 receptors, which operate in orexin regulation of dopamine release in vetral tegmental area [69]. |
1. Akbari E, Naghdi N, Motamedi F. (2006) Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav Brain Res, 173 (1): 47-52. [PMID:16815564]
2. Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, Akerman KE, Kukkonen JP. (2003) Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther, 305 (2): 507-14. [PMID:12606634]
3. Ammoun S, Johansson L, Ekholm ME, Holmqvist T, Danis AS, Korhonen L, Sergeeva OA, Haas HL, Akerman KE, Kukkonen JP. (2006) OX1 orexin receptors activate extracellular signal-regulated kinase in Chinese hamster ovary cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol Endocrinol, 20 (1): 80-99. [PMID:16141359]
4. Ammoun S, Lindholm D, Wootz H, Akerman KE, Kukkonen JP. (2006) G-protein-coupled OX1 orexin/hcrtr-1 hypocretin receptors induce caspase-dependent and -independent cell death through p38 mitogen-/stress-activated protein kinase. J Biol Chem, 281 (2): 834-42. [PMID:16282319]
5. Asahi S, Egashira S, Matsuda M, Iwaasa H, Kanatani A, Ohkubo M, Ihara M, Morishima H. (2003) Development of an orexin-2 receptor selective agonist, [Ala(11), D-Leu(15)]orexin-B. Bioorg Med Chem Lett, 13 (1): 111-3. [PMID:12467628]
6. Backberg M, Hervieu G, Wilson S, Meister B. (2002) Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci, 15: 315-328. [PMID:11849298]
7. Bergman JM, Roecker AJ, Mercer SP, Bednar RA, Reiss DR, Ransom RW, Meacham Harrell C, Pettibone DJ, Lemaire W, Murphy KL et al.. (2008) Proline bis-amides as potent dual orexin receptor antagonists. Bioorg Med Chem Lett, 18 (4): 1425-30. [PMID:18207395]
8. Bingham S, Davey PT, Babbs AJ, Irving EA, Sammons MJ, Wyles M, Jeffrey P, Cutler L, Riba I, Johns A et al.. (2001) Orexin-A, an hypothalamic peptide with analgesic properties. Pain, 92 (1-2): 81-90. [PMID:11323129]
9. Blanco M, López M, GarcIa-Caballero T, Gallego R, Vázquez-Boquete A, Morel G, SeñarIs R, Casanueva F, Diéguez C, Beiras A. (2001) Cellular localization of orexin receptors in human pituitary. J Clin Endocrinol Metab, 86 (7): 1616-9. [PMID:11443222]
10. Bonaventure P, Shelton J, Yun S, Nepomuceno D, Sutton S, Aluisio L, Fraser I, Lord B, Shoblock J, Welty N et al.. (2015) Characterization of JNJ-42847922, a Selective Orexin-2 Receptor Antagonist, as a Clinical Candidate for the Treatment of Insomnia. J Pharmacol Exp Ther, 354 (3): 471-82. [PMID:26177655]
11. Bonaventure P, Yun S, Johnson PL, Shekhar A, Fitz SD, Shireman BT, Lebold TP, Nepomuceno D, Lord B, Wennerholm M et al.. (2015) A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J Pharmacol Exp Ther, 352 (3): 590-601. [PMID:25583879]
12. Boss C, Roch-Brisbare C, Steiner MA, Treiber A, Dietrich H, Jenck F, von Raumer M, Sifferlen T, Brotschi C, Heidmann B et al.. (2014) Structure-activity relationship, biological, and pharmacological characterization of the proline sulfonamide ACT-462206: a potent, brain-penetrant dual orexin 1/orexin 2 receptor antagonist. ChemMedChem, 9 (11): 2486-96. [PMID:25147058]
13. Bourgin P, Huitrón-Résendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. (2000) Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci, 20 (20): 7760-5. [PMID:11027239]
14. Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. (2005) Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA, 102 (52): 19168-73. [PMID:16357203]
15. Caillol M, Aioun J, Baly C, Persuy MA, Salesse R. (2003) Localization of orexins and their receptors in the rat olfactory system: possible modulation of olfactory perception by a neuropeptide synthetized centrally or locally. Brain Res, 960: 48-61. [PMID:12505657]
16. Callander GE, Olorunda M, Monna D, Schuepbach E, Langenegger D, Betschart C, Hintermann S, Behnke D, Cotesta S, Fendt M et al.. (2013) Kinetic properties of "dual" orexin receptor antagonists at OX1R and OX2R orexin receptors. Front Neurosci, 7: 230. [PMID:24376396]
17. Chen J, Randeva HS. (2004) Genomic organization of mouse orexin receptors: characterization of two novel tissue-specific splice variants. Mol Endocrinol, 18 (11): 2790-804. [PMID:15256537]
18. Christopher JA, Aves SJ, Brown J, Errey JC, Klair SS, Langmead CJ, Mace OJ, Mould R, Patel JC, Tehan BG. (2015) Discovery of HTL6641, a dual orexin receptor antagonist with differentiated pharmacodynamic properties. Medicinal Chemistry Communications, 6: 947-955. DOI: 10.1039/C5MD00027K
19. Cox CD, Breslin MJ, Whitman DB, Schreier JD, McGaughey GB, Bogusky MJ, Roecker AJ, Mercer SP, Bednar RA, Lemaire W, Bruno JG, Reiss DR, Harrell CM, Murphy KL, Garson SL, Doran SM, Prueksaritanont T, Anderson WB, Tang C, Roller S, Cabalu TD, Cui D, Hartman GD, Young SD, Koblan KS, Winrow CJ, Renger JJ, Coleman PJ. (2010) Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem, 53 (14): 5320-32. [PMID:20565075]
20. Darker JG, Porter RA, Eggleston DS, Smart D, Brough SJ, Sabido-David C, Jerman JC. (2001) Structure-activity analysis of truncated orexin-A analogues at the orexin-1 receptor. Bioorg Med Chem Lett, 11 (5): 737-40. [PMID:11266181]
21. Di Fabio R, Pellacani A, Faedo S, Roth A, Piccoli L, Gerrard P, Porter RA, Johnson CN, Thewlis K, Donati D et al.. (2011) Discovery process and pharmacological characterization of a novel dual orexin 1 and orexin 2 receptor antagonist useful for treatment of sleep disorders. Bioorg Med Chem Lett, 21 (18): 5562-7. [PMID:21831639]
22. Duxon MS, Stretton J, Starr K, Jones DN, Holland V, Riley G, Jerman J, Brough S, Smart D, Johns A et al.. (2001) Evidence that orexin-A-evoked grooming in the rat is mediated by orexin-1 (OX1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology (Berl.), 153 (2): 203-9. [PMID:11205420]
23. Ehrström M, Levin F, Kirchgessner AL, Schmidt PT, Hilsted LM, Grybäck P, Jacobsson H, Hellström PM, Näslund E. (2005) Stimulatory effect of endogenous orexin A on gastric emptying and acid secretion independent of gastrin. Regul Pept, 132 (1-3): 9-16. [PMID:16125803]
24. Ehrström M, Näslund E, Ma J, Kirchgessner AL, Hellström PM. (2003) Physiological regulation and NO-dependent inhibition of migrating myoelectric complex in the rat small bowel by OXA. Am J Physiol Gastrointest Liver Physiol, 285: G688-G695. [PMID:12816759]
25. El Firar A, Voisin T, Rouyer-Fessard C, Ostuni MA, Couvineau A, Laburthe M. (2009) Discovery of a functional immunoreceptor tyrosine-based switch motif in a 7-transmembrane-spanning receptor: role in the orexin receptor OX1R-driven apoptosis. FASEB J, 23 (12): 4069-80. [PMID:19661287]
26. Fitch TE, Benvenga MJ, Jesudason CD, Zink C, Vandergriff AB, Menezes MM, Schober DA, Rorick-Kehn LM. (2014) LSN2424100: a novel, potent orexin-2 receptor antagonist with selectivity over orexin-1 receptors and activity in an animal model predictive of antidepressant-like efficacy. Front Neurosci, 8: 5. [PMID:24478625]
27. Glen A, Bürli RW, Livermore D, Buffham W, Merison S, Rowland AE, Newman R, Fieldhouse C, Miller DJ, Dawson LA et al.. (2024) Discovery and first-time disclosure of CVN766, an exquisitely selective orexin 1 receptor antagonist. Bioorg Med Chem Lett, 100: 129629. [PMID:38295907]
28. Harris GC, Wimmer M, Aston-Jones G. (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature, 437 (7058): 556-9. [PMID:16100511]
29. Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. (2000) A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept, 96 (1-2): 45-51. [PMID:11102651]
30. Hellmann J, Drabek M, Yin J, Gunera J, Pröll T, Kraus F, Langmead CJ, Hübner H, Weikert D, Kolb P et al.. (2020) Structure-based development of a subtype-selective orexin 1 receptor antagonist. Proc Natl Acad Sci U S A, 117 (30): 18059-18067. [PMID:32669442]
31. Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. (2001) Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience, 103 (3): 777-97. [PMID:11274794]
32. Hirota K, Kushikata T, Kudo M, Kudo T, Smart D, Matsuki A. (2003) Effects of central hypocretin-1 administration on hemodynamic responses in young-adult and middle-aged rats. Brain Res, 981: 143-150. [PMID:12885435]
33. Hoang QV, Bajic D, Yanagisawa M, Nakajima S, Nakajima Y. (2003) Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol, 90 (2): 693-702. [PMID:12702704]
34. Holmqvist T, Akerman KE, Kukkonen JP. (2001) High specificity of human orexin receptors for orexins over neuropeptide Y and other neuropeptides. Neurosci Lett, 305 (3): 177-80. [PMID:11403934]
35. Holmqvist T, Akerman KE, Kukkonen JP. (2002) Orexin signaling in recombinant neuron-like cells. FEBS Lett, 526 (1-3): 11-4. [PMID:12208495]
36. Holmqvist T, Johansson L, Ostman M, Ammoun S, Akerman KE, Kukkonen JP. (2005) OX1 orexin receptors couple to adenylyl cyclase regulation via multiple mechanisms. J Biol Chem, 280 (8): 6570-9. [PMID:15611118]
37. Hong C, Byrne NJ, Zamlynny B, Tummala S, Xiao L, Shipman JM, Partridge AT, Minnick C, Breslin MJ, Rudd MT et al.. (2021) Structures of active-state orexin receptor 2 rationalize peptide and small-molecule agonist recognition and receptor activation. Nat Commun, 12 (1): 815. [PMID:33547286]
38. Iio K, Hashimoto K, Nagumo Y, Amezawa M, Hasegawa T, Yamamoto N, Kutsumura N, Takeuchi K, Ishikawa Y, Yamamoto H et al.. (2023) Design and Synthesis of Orexin 1 Receptor-Selective Agonists. J Med Chem, 66 (8): 5453-5464. [PMID:37043436]
39. Irukayama-Tomobe Y, Ogawa Y, Tominaga H, Ishikawa Y, Hosokawa N, Ambai S, Kawabe Y, Uchida S, Nakajima R, Saitoh T et al.. (2017) Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci USA, 114 (22): 5731-5736. [PMID:28507129]
40. Ishikawa T, Hara H, Kawano A, Tohyama K, Kajita Y, Miyanohana Y, Koike T, Kimura H. (2023) TAK-994, a Novel Orally Available Brain-Penetrant Orexin 2 Receptor-Selective Agonist, Suppresses Fragmentation of Wakefulness and Cataplexy-Like Episodes in Mouse Models of Narcolepsy. J Pharmacol Exp Ther, 385 (3): 193-204. [PMID:37001988]
41. Johansson L, Ekholm ME, Kukkonen JP. (2007) Regulation of OX1 orexin/hypocretin receptor-coupling to phospholipase C by Ca2+ influx. Br J Pharmacol, 150 (1): 97-104. [PMID:17115071]
42. Johansson L, Ekholm ME, Kukkonen JP. (2008) Multiple phospholipase activation by OX(1) orexin/hypocretin receptors. Cell Mol Life Sci, 65 (12): 1948-56. [PMID:18488139]
43. Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Träskman-Bendz L, Goddard AW, Brundin L, Shekhar A. (2010) A key role for orexin in panic anxiety. Nat Med, 16 (1): 111-5. [PMID:20037593]
44. Jones DN, Gartlon J, Parker F, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Hatcher JP, Johns A et al.. (2001) Effects of centrally administered orexin-B and orexin-A: a role for orexin-1 receptors in orexin-B-induced hyperactivity. Psychopharmacology (Berl.), 153 (2): 210-8. [PMID:11205421]
45. Jäntti M, Putula J, Somerharju P, Frohman M, Kukkonen J. (2012) OX(1) orexin/hypocretin receptor activation of phospholipase D. Br J Pharmacol, 165 (4b): 1109-23. [PMID:21718304]
46. Jäntti MH, Putula J, Turunen PM, Näsman J, Reijonen S, Lindqvist C, Kukkonen JP. (2013) Autocrine endocannabinoid signaling through CB1 receptors potentiates OX1 orexin receptor signaling. Mol Pharmacol, 83 (3): 621-32. [PMID:23233488]
47. Kukkonen JP. (2013) Physiology of the orexinergic/hypocretinergic system: a revisit in 2012. Am J Physiol, Cell Physiol, 304 (1): C2-32. [PMID:23034387]
48. Kukkonen JP. (2016) G-protein inhibition profile of the reported Gq/11 inhibitor UBO-QIC. Biochem Biophys Res Commun, 469 (1): 101-7. [PMID:26614908]
49. Kukkonen JP. (2016) G-protein-dependency of orexin/hypocretin receptor signalling in recombinant Chinese hamster ovary cells. Biochem Biophys Res Commun, 476 (4): 379-85. [PMID:27237973]
50. Kukkonen JP. (2016) OX2 orexin/hypocretin receptor signal transduction in recombinant Chinese hamster ovary cells. Cell Signal, 28 (2): 51-60. [PMID:26582739]
51. Kukkonen JP. (2017) Orexin/Hypocretin Signaling. Curr Top Behav Neurosci, 33: 17-50. [PMID:27909990]
52. Kukkonen JP, Akerman KE. (2001) Orexin receptors couple to Ca2+ channels different from store-operated Ca2+ channels. Neuroreport, 12 (9): 2017-20. [PMID:11435939]
53. Kukkonen JP, Leonard CS. (2014) Orexin/hypocretin receptor signalling cascades. Br J Pharmacol, 171 (2): 314-31. [PMID:23902572]
54. Kunii K, Yamanaka A, Nambu T, Matsuzaki I, Goto K, Sakurai T. (1999) Orexins/hypocretins regulate drinking behaviour. Brain Res, 842 (1): 256-61. [PMID:10526122]
55. Langmead CJ, Jerman JC, Brough SJ, Scott C, Porter RA, Herdon HJ. (2004) Characterisation of the binding of [3H]-SB-674042, a novel nonpeptide antagonist, to the human orexin-1 receptor. Br J Pharmacol, 141 (2): 340-6. [PMID:14691055]
56. Larsson KP, Peltonen HM, Bart G, Louhivuori LM, Penttonen A, Antikainen M, Kukkonen JP, Akerman KE. (2005) Orexin-A-induced Ca2+ entry: evidence for involvement of trpc channels and protein kinase C regulation. J Biol Chem, 280 (3): 1771-81. [PMID:15537648]
57. Leonard CS, Kukkonen JP. (2014) Orexin/hypocretin receptor signalling: a functional perspective. Br J Pharmacol, 171 (2): 294-313. [PMID:23848055]
58. Lund PE, Shariatmadari R, Uustare A, Detheux M, Parmentier M, Kukkonen JP, Akerman KE. (2000) The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J Biol Chem, 275 (40): 30806-12. [PMID:10880509]
59. López M, Señarís R, Gallego R, García-Caballero T, Lago F, Seoane L, Casanueva F, Diéguez C. (1999) Orexin receptors are expressed in the adrenal medulla of the rat. Endocrinology, 140 (12): 5991-4. [PMID:10579367]
60. Magga J, Bart G, Oker-Blom C, Kukkonen JP, Akerman KE, Näsman J. (2006) Agonist potency differentiates G protein activation and Ca2+ signalling by the orexin receptor type 1. Biochem Pharmacol, 71 (6): 827-36. [PMID:16430869]
61. Malherbe P, Borroni E, Gobbi L, Knust H, Nettekoven M, Pinard E, Roche O, Rogers-Evans M, Wettstein JG, Moreau JL. (2009) Biochemical and behavioural characterization of EMPA, a novel high-affinity, selective antagonist for the OX(2) receptor. Br J Pharmacol, 156 (8): 1326-41. [PMID:19751316]
62. Malherbe P, Borroni E, Pinard E, Wettstein JG, Knoflach F. (2009) Biochemical and electrophysiological characterization of almorexant, a dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist: comparison with selective OX1 and OX2 antagonists. Mol Pharmacol, 76 (3): 618-31. [PMID:19542319]
63. Malherbe P, Roche O, Marcuz A, Kratzeisen C, Wettstein JG, Bissantz C. (2010) Mapping the binding pocket of dual antagonist almorexant to human orexin 1 and orexin 2 receptors: comparison with the selective OX1 antagonist SB-674042 and the selective OX2 antagonist N-ethyl-2-[(6-methoxy-pyridin-3-yl)-(toluene-2-sulfonyl)-amino]-N-pyridin-3-ylmethyl-acetamide (EMPA). Mol Pharmacol, 78 (1): 81-93. [PMID:20404073]
64. McAtee LC, Sutton SW, Rudolph DA, Li X, Aluisio LE, Phuong VK, Dvorak CA, Lovenberg TW, Carruthers NI, Jones TK. (2004) Novel substituted 4-phenyl-[1,3]dioxanes: potent and selective orexin receptor 2 (OX(2)R) antagonists. Bioorg Med Chem Lett, 14 (16): 4225-9. [PMID:15261275]
65. Milasta S, Evans NA, Ormiston L, Wilson S, Lefkowitz RJ, Milligan G. (2005) The sustainability of interactions between the orexin-1 receptor and beta-arrestin-2 is defined by a single C-terminal cluster of hydroxy amino acids and modulates the kinetics of ERK MAPK regulation. Biochem J, 387 (Pt 3): 573-84. [PMID:15683363]
66. Mitsukawa K, Kimura H. (2022) Orexin 2 receptor (OX2R) protein distribution measured by autoradiography using radiolabeled OX2R-selective antagonist EMPA in rodent brain and peripheral tissues. Sci Rep, 12 (1): 8473. [PMID:35589803]
67. Mould R, Brown J, Marshall FH, Langmead CJ. (2014) Binding kinetics differentiates functional antagonism of orexin-2 receptor ligands. Br J Pharmacol, 171 (2): 351-63. [PMID:23692283]
68. Nagahara T, Saitoh T, Kutsumura N, Irukayama-Tomobe Y, Ogawa Y, Kuroda D, Gouda H, Kumagai H, Fujii H, Yanagisawa M et al.. (2015) Design and Synthesis of Non-Peptide, Selective Orexin Receptor 2 Agonists. J Med Chem, 58 (20): 7931-7. [PMID:26267383]
69. Navarro G, Quiroz C, Moreno-Delgado D, Sierakowiak A, McDowell K, Moreno E, Rea W, Cai NS, Aguinaga D, Howell LA et al.. (2015) Orexin-corticotropin-releasing factor receptor heteromers in the ventral tegmental area as targets for cocaine. J Neurosci, 35 (17): 6639-53. [PMID:25926444]
70. Nowak KW, Strowski MZ, Switonska MM, Kaczmarek P, Singh V, Fabis M, Mackowiak P, Nowak M, Malendowicz LK. (2005) Evidence that orexins A and B stimulate insulin secretion from rat pancreatic islets via both receptor subtypes. Int J Mol Med, 15 (6): 969-72. [PMID:15870901]
71. Näsman J, Bart G, Larsson K, Louhivuori L, Peltonen H, Akerman KE. (2006) The orexin OX1 receptor regulates Ca2+ entry via diacylglycerol-activated channels in differentiated neuroblastoma cells. J Neurosci, 26 (42): 10658-66. [PMID:17050705]
72. Okumura T, Takeuchi S, Motomura W, Yamada H, Egashira Si S, Asahi S, Kanatani A, Ihara M, Kohgo Y. (2001) Requirement of intact disulfide bonds in orexin-A-induced stimulation of gastric acid secretion that is mediated by OX1 receptor activation. Biochem Biophys Res Commun, 280 (4): 976-81. [PMID:11162621]
73. Peltonen HM, Magga JM, Bart G, Turunen PM, Antikainen MS, Kukkonen JP, Akerman KE. (2009) Involvement of TRPC3 channels in calcium oscillations mediated by OX(1) orexin receptors. Biochem Biophys Res Commun, 385 (3): 408-12. [PMID:19464259]
74. Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, Jerman JC, Brough SJ, Coldwell M, Smart D et al.. (2001) 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett, 11 (14): 1907-10. [PMID:11459658]
75. Putula J, Pihlajamaa T, Kukkonen JP. (2014) Calcium affects OX1 orexin (hypocretin) receptor responses by modifying both orexin binding and the signal transduction machinery. Br J Pharmacol, 171 (24): 5816-28. [PMID:25132134]
76. Putula J, Turunen PM, Jäntti MH, Ekholm ME, Kukkonen JP. (2011) Agonist ligand discrimination by the two orexin receptors depends on the expression system. Neurosci Lett, 494 (1): 57-60. [PMID:21362456]
77. Rappas M, Ali AAE, Bennett KA, Brown JD, Bucknell SJ, Congreve M, Cooke RM, Cseke G, de Graaf C, Doré AS et al.. (2020) Comparison of Orexin 1 and Orexin 2 Ligand Binding Modes Using X-ray Crystallography and Computational Analysis. J Med Chem, 63 (4): 1528-1543. [PMID:31860301]
78. Rinne MK, Leino TO, Turku A, Turunen PM, Steynen Y, Xhaard H, Wallén EAA, Kukkonen JP. (2018) Pharmacological characterization of the orexin/hypocretin receptor agonist Nag 26. Eur J Pharmacol, 837: 137-144. [PMID:30194937]
79. Roch C, Bergamini G, Steiner MA, Clozel M. (2021) Nonclinical pharmacology of daridorexant: a new dual orexin receptor antagonist for the treatment of insomnia. Psychopharmacology (Berl), 238 (10): 2693-2708. [PMID:34415378]
80. Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Upton N, Porter RA, Johns A, Blundell JE. (2001) SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci, 13 (7): 1444-52. [PMID:11298806]
81. Roecker AJ, Mercer SP, Schreier JD, Cox CD, Fraley ME, Steen JT, Lemaire W, Bruno JG, Harrell CM, Garson SL et al.. (2014) Discovery of 5''-chloro-N-[(5,6-dimethoxypyridin-2-yl)methyl]-2,2':5',3''-terpyridine-3'-carboxamide (MK-1064): a selective orexin 2 receptor antagonist (2-SORA) for the treatment of insomnia. ChemMedChem, 9 (2): 311-22. [PMID:24376006]
82. Roecker AJ, Reger TS, Mattern MC, Mercer SP, Bergman JM, Schreier JD, Cube RV, Cox CD, Li D, Lemaire W et al.. (2014) Discovery of MK-3697: a selective orexin 2 receptor antagonist (2-SORA) for the treatment of insomnia. Bioorg Med Chem Lett, 24 (20): 4884-90. [PMID:25248679]
83. Rouet-Benzineb P, Rouyer-Fessard C, Jarry A, Avondo V, Pouzet C, Yanagisawa M, Laboisse C, Laburthe M, Voisin T. (2004) Orexins acting at native OX(1) receptor in colon cancer and neuroblastoma cells or at recombinant OX(1) receptor suppress cell growth by inducing apoptosis. J Biol Chem, 279 (44): 45875-86. [PMID:15310763]
84. Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S et al.. (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell, 92 (4): 573-85. [PMID:9491897]
85. Salvadore G, Bonaventure P, Shekhar A, Johnson PL, Lord B, Shireman BT, Lebold TP, Nepomuceno D, Dugovic C, Brooks S et al.. (2020) Translational evaluation of novel selective orexin-1 receptor antagonist JNJ-61393215 in an experimental model for panic in rodents and humans. Transl Psychiatry, 10 (1): 308. [PMID:32895369]
86. Savaskan E, Müller-Spahn F, Meier F, Wirz-Justice A, Meyer P. (2004) Orexins and their receptors in the human retina. Pathobiology, 71 (4): 211-6. [PMID:15263810]
87. Schneeberger M, Brice NL, Pellegrino K, Parolari L, Shaked JT, Page KJ, Marchildon F, Barrows DW, Carroll TS, Topilko T et al.. (2022) Pharmacological targeting of glutamatergic neurons within the brainstem for weight reduction. Nat Metab, 4 (11): 1495-1513. [PMID:36411386]
88. Smart D, Jerman JC, Brough SJ, Rushton SL, Murdock PR, Jewitt F, Elshourbagy NA, Ellis CE, Middlemiss DN, Brown F. (1999) Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol, 128 (1): 1-3. [PMID:10498827]
89. Steiner MA, Gatfield J, Brisbare-Roch C, Dietrich H, Treiber A, Jenck F, Boss C. (2013) Discovery and characterization of ACT-335827, an orally available, brain penetrant orexin receptor type 1 selective antagonist. ChemMedChem, 8 (6): 898-903. [PMID:23589487]
90. Tran DT, Bonaventure P, Hack M, Mirzadegan T, Dvorak C, Letavic M, Carruthers N, Lovenberg T, Sutton SW. (2011) Chimeric, mutant orexin receptors show key interactions between orexin receptors, peptides and antagonists. Eur J Pharmacol, 667 (1-3): 120-8. [PMID:21679703]
91. Treiber A, de Kanter R, Roch C, Gatfield J, Boss C, von Raumer M, Schindelholz B, Muehlan C, van Gerven J, Jenck F. (2017) The Use of Physiology-Based Pharmacokinetic and Pharmacodynamic Modeling in the Discovery of the Dual Orexin Receptor Antagonist ACT-541468. J Pharmacol Exp Ther, 362 (3): 489-503. [PMID:28663311]
92. Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. (1998) Distribution of orexin receptor mRNA in the rat brain. FEBS Lett, 438: 71-75. [PMID:9821961]
93. Turunen PM, Ekholm ME, Somerharju P, Kukkonen JP. (2010) Arachidonic acid release mediated by OX1 orexin receptors. Br J Pharmacol, 159 (1): 212-21. [PMID:20002100]
94. Turunen PM, Jäntti MH, Kukkonen JP. (2012) OX1 orexin/hypocretin receptor signaling through arachidonic acid and endocannabinoid release. Mol Pharmacol, 82 (2): 156-67. [PMID:22550093]
95. Voisin T, El Firar A, Fasseu M, Rouyer-Fessard C, Descatoire V, Walker F, Paradis V, Bedossa P, Henin D, Lehy T et al.. (2011) Aberrant expression of OX1 receptors for orexins in colon cancers and liver metastases: an openable gate to apoptosis. Cancer Res, 71 (9): 3341-51. [PMID:21415167]
96. Voisin T, El Firar A, Rouyer-Fessard C, Gratio V, Laburthe M. (2008) A hallmark of immunoreceptor, the tyrosine-based inhibitory motif ITIM, is present in the G protein-coupled receptor OX1R for orexins and drives apoptosis: a novel mechanism. FASEB J, 22 (6): 1993-2002. [PMID:18198212]
97. Wayner MJ, Armstrong DL, Phelix CF, Oomura Y. (2004) Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides, 25 (6): 991-6. [PMID:15203246]
98. Winrow CJ, Gotter AL, Cox CD, Tannenbaum PL, Garson SL, Doran SM, Breslin MJ, Schreier JD, Fox SV, Harrell CM et al.. (2012) Pharmacological characterization of MK-6096 - A dual orexin receptor antagonist for insomnia. Neuropharmacology, 62 (2): 978-87. [PMID:22019562]
99. Yamamoto T, Nozaki-Taguchi N, Chiba T. (2002) Analgesic effect of intrathecally administered orexin-A in the rat formalin test and in the rat hot plate test. Br J Pharmacol, 137 (2): 170-6. [PMID:12208773]
100. Yin J, Babaoglu K, Brautigam CA, Clark L, Shao Z, Scheuermann TH, Harrell CM, Gotter AL, Roecker AJ, Winrow CJ et al.. (2016) Structure and ligand-binding mechanism of the human OX1 and OX2 orexin receptors. Nat Struct Mol Biol, 23 (4): 293-9. [PMID:26950369]
101. Yoshida Y, Naoe Y, Terauchi T, Ozaki F, Doko T, Takemura A, Tanaka T, Sorimachi K, Beuckmann CT, Suzuki M et al.. (2015) Discovery of (1R,2S)-2-{[(2,4-Dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide (E2006): A Potent and Efficacious Oral Orexin Receptor Antagonist. J Med Chem, 58 (11): 4648-64. [PMID:25953512]
102. Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. (2005) Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol, 98 (4): 1387-95. [PMID:15557013]
103. Yukitake H, Fujimoto T, Ishikawa T, Suzuki A, Shimizu Y, Rikimaru K, Ito M, Suzuki M, Kimura H. (2019) TAK-925, an orexin 2 receptor-selective agonist, shows robust wake-promoting effects in mice. Pharmacol Biochem Behav, 187: 172794. [PMID:31654653]
104. Zhang D, Perrey DA, Decker AM, Langston TL, Mavanji V, Harris DL, Kotz CM, Zhang Y. (2021) Discovery of Arylsulfonamides as Dual Orexin Receptor Agonists. J Med Chem, 64 (12): 8806-8825. [PMID:34101446]
105. Zhu Y, Miwa Y, Yamanaka A, Yada T, Shibahara M, Abe Y, Sakurai T, Goto K. (2003) Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci, 92: 259-266. [PMID:12890892]