1. Achat-Mendes C, Lynch LJ, Sullivan KA, Vallender EJ, Miller GM. (2012) Augmentation of methamphetamine-induced behaviors in transgenic mice lacking the trace amine-associated receptor 1.
Pharmacol Biochem Behav, 101 (2): 201-7.
[PMID:22079347]
2. Adriaenssens A, Lam BY, Billing L, Skeffington K, Sewing S, Reimann F, Gribble F. (2015) A Transcriptome-Led Exploration of Molecular Mechanisms Regulating Somatostatin-Producing D-Cells in the Gastric Epithelium.
Endocrinology, 156 (11): 3924-36.
[PMID:26241122]
3. Alvarsson A, Zhang X, Stan TL, Schintu N, Kadkhodaei B, Millan MJ, Perlmann T, Svenningsson P. (2015) Modulation by Trace Amine-Associated Receptor 1 of Experimental Parkinsonism, L-DOPA Responsivity, and Glutamatergic Neurotransmission.
J Neurosci, 35 (41): 14057-69.
[PMID:26468205]
4. Babusyte A, Kotthoff M, Fiedler J, Krautwurst D. (2013) Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2.
J Leukoc Biol, 93 (3): 387-94.
[PMID:23315425]
5. Barak LS, Salahpour A, Zhang X, Masri B, Sotnikova TD, Ramsey AJ, Violin JD, Lefkowitz RJ, Caron MG, Gainetdinov RR. (2008) Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor.
Mol Pharmacol, 74 (3): 585-594.
[PMID:18524885]
6. Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S et al.. (2001) Trace amines: identification of a family of mammalian G protein-coupled receptors.
Proc Natl Acad Sci USA, 98 (16): 8966-71.
[PMID:11459929]
7. Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, Pinard A, Buchy D, Gassmann M, Hoener MC et al.. (2009) The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system.
Proc Natl Acad Sci USA, 106 (47): 20081-6.
[PMID:19892733]
8. Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL et al.. (2001) Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor.
Mol Pharmacol, 60 (6): 1181-8.
[PMID:11723224]
9. Chiellini G, Frascarelli S, Ghelardoni S, Carnicelli V, Tobias SC, DeBarber A, Brogioni S, Ronca-Testoni S, Cerbai E, Grandy DK et al.. (2007) Cardiac effects of 3-iodothyronamine: a new aminergic system modulating cardiac function.
FASEB J, 21 (7): 1597-608.
[PMID:17284482]
10. Cisneros IE, Ghorpade A. (2014) Methamphetamine and HIV-1-induced neurotoxicity: role of trace amine associated receptor 1 cAMP signaling in astrocytes.
Neuropharmacology, 85: 499-507.
[PMID:24950453]
11. D'Andrea G, Terrazzino S, Fortin D, Farruggio A, Rinaldi L, Leon A. (2003) HPLC electrochemical detection of trace amines in human plasma and platelets and expression of mRNA transcripts of trace amine receptors in circulating leukocytes.
Neurosci Lett, 346 (1-2): 89-92.
[PMID:12850555]
12. Dedic N, Jones PG, Hopkins SC, Lew R, Shao L, Campbell JE, Spear KL, Large TH, Campbell UC, Hanania T et al.. (2019) SEP-363856, a Novel Psychotropic Agent with a Unique, Non-D2 Receptor Mechanism of Action.
J Pharmacol Exp Ther, 371 (1): 1-14.
[PMID:31371483]
13. Di Cara B, Maggio R, Aloisi G, Rivet JM, Lundius EG, Yoshitake T, Svenningsson P, Brocco M, Gobert A, De Groote L et al.. (2011) Genetic deletion of trace amine 1 receptors reveals their role in auto-inhibiting the actions of ecstasy (MDMA).
J Neurosci, 31 (47): 16928-40.
[PMID:22114263]
14. Espinoza S, Ghisi V, Emanuele M, Leo D, Sukhanov I, Sotnikova TD, Chieregatti E, Gainetdinov RR. (2015) Postsynaptic D2 dopamine receptor supersensitivity in the striatum of mice lacking TAAR1.
Neuropharmacology, 93: 308-13.
[PMID:25721394]
15. Espinoza S, Lignani G, Caffino L, Maggi S, Sukhanov I, Leo D, Mus L, Emanuele M, Ronzitti G, Harmeier A et al.. (2015) TAAR1 Modulates Cortical Glutamate NMDA Receptor Function.
Neuropsychopharmacology, 40 (9): 2217-27.
[PMID:25749299]
16. Espinoza S, Salahpour A, Masri B, Sotnikova TD, Messa M, Barak LS, Caron MG, Gainetdinov RR. (2011) Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor.
Mol Pharmacol, 80 (3): 416-25.
[PMID:21670104]
17. Fehler M, Broadley KJ, Ford WR, Kidd EJ. (2010) Identification of trace-amine-associated receptors (TAAR) in the rat aorta and their role in vasoconstriction by β-phenylethylamine.
Naunyn Schmiedebergs Arch Pharmacol, 382 (4): 385-98.
[PMID:20809238]
18. Galley G, Beurier A, Décoret G, Goergler A, Hutter R, Mohr S, Pähler A, Schmid P, Türck D, Unger R et al.. (2016) Discovery and Characterization of 2-Aminooxazolines as Highly Potent, Selective, and Orally Active TAAR1 Agonists.
ACS Med Chem Lett, 7 (2): 192-7.
[PMID:26985297]
19. Galley G, Hoener M, Norcross R, Pflieger P. (2017) 5-ethyl-4-methyl-pyrazole-3-carboxamide derivative having activity as agonist of taar.
Patent number: WO2017157873A1. Assignee: Hoffmann-La Roche. Priority date: 17/03/2016. Publication date: 21/09/2017.
20. Galley G, Stalder H, Goergler A, Hoener MC, Norcross RD. (2012) Optimisation of imidazole compounds as selective TAAR1 agonists: discovery of RO5073012.
Bioorg Med Chem Lett, 22 (16): 5244-8.
[PMID:22795332]
21. Gozal EA, O'Neill BE, Sawchuk MA, Zhu H, Halder M, Chou CC, Hochman S. (2014) Anatomical and functional evidence for trace amines as unique modulators of locomotor function in the mammalian spinal cord.
Front Neural Circuits, 8: 134.
[PMID:25426030]
22. Hart ME, Suchland KL, Miyakawa M, Bunzow JR, Grandy DK, Scanlan TS. (2006) Trace amine-associated receptor agonists: synthesis and evaluation of thyronamines and related analogues.
J Med Chem, 49 (3): 1101-12.
[PMID:16451074]
23. Hu LA, Zhou T, Ahn J, Wang S, Zhou J, Hu Y, Liu Q. (2009) Human and mouse trace amine-associated receptor 1 have distinct pharmacology towards endogenous monoamines and imidazoline receptor ligands.
Biochem J, 424 (1): 39-45.
[PMID:19725810]
24. Kleinau G, Pratzka J, Nürnberg D, Grüters A, Führer-Sakel D, Krude H, Köhrle J, Schöneberg T, Biebermann H. (2011) Differential modulation of Beta-adrenergic receptor signaling by trace amine-associated receptor 1 agonists.
PLoS ONE, 6 (10): e27073.
[PMID:22073124]
25. Lam VM, Espinoza S, Gerasimov AS, Gainetdinov RR, Salahpour A. (2015) In-vivo pharmacology of Trace-Amine Associated Receptor 1.
Eur J Pharmacol, 763 (Pt B): 136-42.
[PMID:26093041]
26. Leo D, Mus L, Espinoza S, Hoener MC, Sotnikova TD, Gainetdinov RR. (2014) Taar1-mediated modulation of presynaptic dopaminergic neurotransmission: role of D2 dopamine autoreceptors.
Neuropharmacology, 81: 283-91.
[PMID:24565640]
27. Lewin AH, Miller GM, Gilmour B. (2011) Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class.
Bioorg Med Chem, 19 (23): 7044-8.
[PMID:22037049]
28. Lewin AH, Navarro HA, Mascarella SW. (2008) Structure-activity correlations for beta-phenethylamines at human trace amine receptor 1.
Bioorg Med Chem, 16 (15): 7415-23.
[PMID:18602830]
29. Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC. (2005) Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors.
Genomics, 85 (3): 372-85.
[PMID:15718104]
30. Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein JG, Borroni E, Moreau JL et al.. (2008) Trace amine-associated receptor 1 modulates dopaminergic activity.
J Pharmacol Exp Ther, 324 (3): 948-56.
[PMID:18083911]
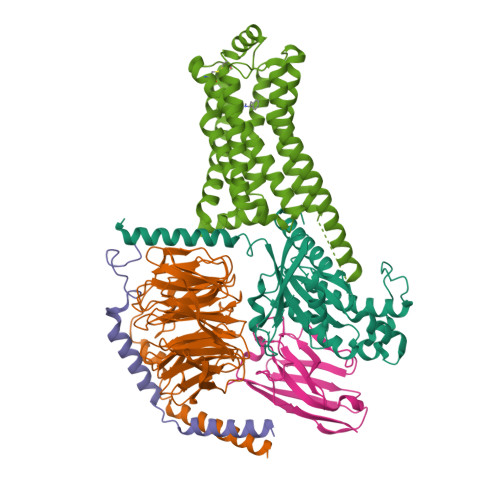
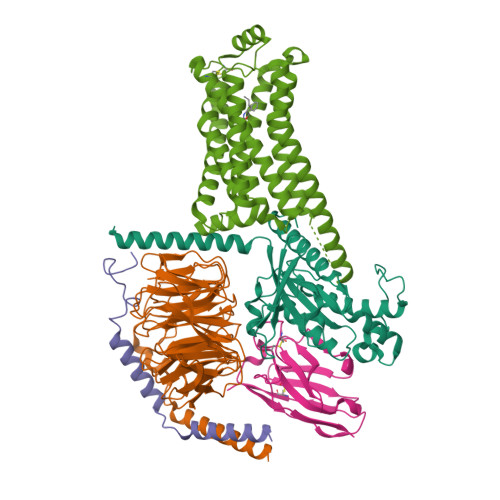
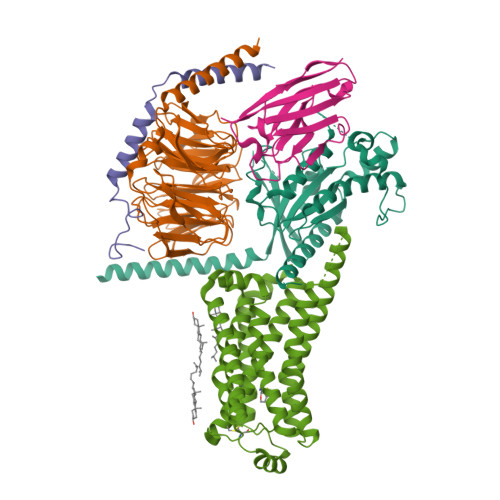
31. Liu H, Zheng Y, Wang Y, Wang Y, He X, Xu P, Huang S, Yuan Q, Zhang X, Wang L et al.. (2023) Recognition of methamphetamine and other amines by trace amine receptor TAAR1.
Nature, 624 (7992): 663-671.
[PMID:37935377]
32. Liu J, Johnson B, Wu R, Seaman Jr R, Vu J, Zhu Q, Zhang Y, Li JX. (2020) TAAR1 agonists attenuate extended-access cocaine self-administration and yohimbine-induced reinstatement of cocaine-seeking.
Br J Pharmacol, 177 (15): 3403-3414.
[PMID:32246467]
33. Liu JF, Thorn DA, Zhang Y, Li JX. (2016) Effects of Trace Amine-associated Receptor 1 Agonists on the Expression, Reconsolidation, and Extinction of Cocaine Reward Memory.
Int J Neuropsychopharmacol, 19 (7).
[PMID:26822713]
34. Liu X, Grandy DK, Janowsky A. (2014) Ractopamine, a livestock feed additive, is a full agonist at trace amine-associated receptor 1.
J Pharmacol Exp Ther, 350 (1): 124-9.
[PMID:24799633]
35. Lynch LJ, Sullivan KA, Vallender EJ, Rowlett JK, Platt DM, Miller GM. (2013) Trace amine associated receptor 1 modulates behavioral effects of ethanol.
Subst Abuse, 7: 117-26.
[PMID:23861588]
36. Maguire JJ, Parker WA, Foord SM, Bonner TI, Neubig RR, Davenport AP. (2009) International Union of Pharmacology. LXXII. Recommendations for trace amine receptor nomenclature.
Pharmacol Rev, 61 (1): 1-8.
[PMID:19325074]
37. Miller GM. (2011) The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity.
J Neurochem, 116 (2): 164-76.
[PMID:21073468]
38. Miller GM, Verrico CD, Jassen A, Konar M, Yang H, Panas H, Bahn M, Johnson R, Madras BK. (2005) Primate trace amine receptor 1 modulation by the dopamine transporter.
J Pharmacol Exp Ther, 313 (3): 983-94.
[PMID:15764732]
39. Miyakawa M, Scanlan TS. (2006) Synthesis of [125I]-, [2H]-, and [3H]-labelled 3-iodothyronamine (T1AM).
Synthetic Communications, 36: 891-902.
40. Navarro HA, Gilmour BP, Lewin AH. (2006) A rapid functional assay for the human trace amine-associated receptor 1 based on the mobilization of internal calcium.
J Biomol Screen, 11 (6): 688-93.
[PMID:16831861]
41. Nelson DA, Tolbert MD, Singh SJ, Bost KL. (2007) Expression of neuronal trace amine-associated receptor (Taar) mRNAs in leukocytes.
J Neuroimmunol, 192 (1-2): 21-30.
[PMID:17900709]
42. Pei Y, Asif-Malik A, Canales JJ. (2016) Trace Amines and the Trace Amine-Associated Receptor 1: Pharmacology, Neurochemistry, and Clinical Implications.
Front Neurosci, 10: 148.
[PMID:27092049]
43. Pei Y, Mortas P, Hoener MC, Canales JJ. (2015) Selective activation of the trace amine-associated receptor 1 decreases cocaine's reinforcing efficacy and prevents cocaine-induced changes in brain reward thresholds.
Prog Neuropsychopharmacol Biol Psychiatry, 63: 70-5.
[PMID:26048337]
44. Piehl S, Hoefig CS, Scanlan TS, Köhrle J. (2011) Thyronamines--past, present, and future.
Endocr Rev, 32 (1): 64-80.
[PMID:20880963]
45. Raab S, Wang H, Uhles S, Cole N, Alvarez-Sanchez R, Künnecke B, Ullmer C, Matile H, Bedoucha M, Norcross RD et al.. (2016) Incretin-like effects of small molecule trace amine-associated receptor 1 agonists.
Mol Metab, 5 (1): 47-56.
[PMID:26844206]
46. Reese EA, Bunzow JR, Arttamangkul S, Sonders MS, Grandy DK. (2007) Trace amine-associated receptor 1 displays species-dependent stereoselectivity for isomers of methamphetamine, amphetamine, and para-hydroxyamphetamine.
J Pharmacol Exp Ther, 321 (1): 178-86.
[PMID:17218486]
47. Regard JB, Kataoka H, Cano DA, Camerer E, Yin L, Zheng YW, Scanlan TS, Hebrok M, Coughlin SR. (2007) Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion.
J Clin Invest, 117 (12): 4034-43.
[PMID:17992256]
48. Revel FG, Meyer CA, Bradaia A, Jeanneau K, Calcagno E, André CB, Haenggi M, Miss MT, Galley G, Norcross RD et al.. (2012) Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine.
Neuropsychopharmacology, 37 (12): 2580-92.
[PMID:22763617]
49. Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA et al.. (2011) TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity.
Proc Natl Acad Sci USA, 108 (20): 8485-90.
[PMID:21525407]
50. Revel FG, Moreau JL, Gainetdinov RR, Ferragud A, Velázquez-Sánchez C, Sotnikova TD, Morairty SR, Harmeier A, Groebke Zbinden K, Norcross RD et al.. (2012) Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics.
Biol Psychiatry, 72 (11): 934-42.
[PMID:22705041]
51. Revel FG, Moreau JL, Pouzet B, Mory R, Bradaia A, Buchy D, Metzler V, Chaboz S, Groebke Zbinden K, Galley G et al.. (2013) A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight.
Mol Psychiatry, 18 (5): 543-56.
[PMID:22641180]
52. Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, Frascarelli S, Crossley DA, Bunzow JR, Ronca-Testoni S et al.. (2004) 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone.
Nat Med, 10 (6): 638-42.
[PMID:15146179]
53. Smith SB, Maixner DW, Fillingim RB, Slade G, Gracely RH, Ambrose K, Zaykin DV, Hyde C, John S, Tan K et al.. (2012) Large candidate gene association study reveals genetic risk factors and therapeutic targets for fibromyalgia.
Arthritis Rheum, 64 (2): 584-93.
[PMID:21905019]
54. Sotnikova TD, Caron MG, Gainetdinov RR. (2009) Trace amine-associated receptors as emerging therapeutic targets.
Mol Pharmacol, 76 (2): 229-35.
[PMID:19389919]
55. Sotnikova TD, Zorina OI, Ghisi V, Caron MG, Gainetdinov RR. (2008) Trace amine associated receptor 1 and movement control.
Parkinsonism Relat Disord, 14 Suppl 2: S99-102.
[PMID:18585080]
56. Stalder H, Hoener MC, Norcross RD. (2011) Selective antagonists of mouse trace amine-associated receptor 1 (mTAAR1): discovery of EPPTB (RO5212773).
Bioorg Med Chem Lett, 21 (4): 1227-31.
[PMID:21237643]
57. Sukhanov I, Espinoza S, Yakovlev DS, Hoener MC, Sotnikova TD, Gainetdinov RR. (2014) TAAR1-dependent effects of apomorphine in mice.
Int J Neuropsychopharmacol, 17 (10): 1683-93.
[PMID:24925023]
58. Szumska J, Qatato M, Rehders M, Führer D, Biebermann H, Grandy DK, Köhrle J, Brix K. (2015) Trace Amine-Associated Receptor 1 Localization at the Apical Plasma Membrane Domain of Fisher Rat Thyroid Epithelial Cells Is Confined to Cilia.
Eur Thyroid J, 4 (Suppl 1): 30-41.
[PMID:26601071]
59. Tan ES, Groban ES, Jacobson MP, Scanlan TS. (2008) Toward deciphering the code to aminergic G protein-coupled receptor drug design.
Chem Biol, 15 (4): 343-53.
[PMID:18420141]
60. Tan ES, Miyakawa M, Bunzow JR, Grandy DK, Scanlan TS. (2007) Exploring the structure-activity relationship of the ethylamine portion of 3-iodothyronamine for rat and mouse trace amine-associated receptor 1.
J Med Chem, 50 (12): 2787-98.
[PMID:17497842]
61. Wainscott DB, Little SP, Yin T, Tu Y, Rocco VP, He JX, Nelson DL. (2007) Pharmacologic characterization of the cloned human trace amine-associated receptor1 (TAAR1) and evidence for species differences with the rat TAAR1.
J Pharmacol Exp Ther, 320 (1): 475-85.
[PMID:17038507]
62. Wasik AM, Millan MJ, Scanlan T, Barnes NM, Gordon J. (2012) Evidence for functional trace amine associated receptor-1 in normal and malignant B cells.
Leuk Res, 36 (2): 245-9.
[PMID:22036195]
63. Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P, Branchek T, Gerald CP. (2007) The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia.
Genes Brain Behav, 6 (7): 628-39.
[PMID:17212650]
64. Xie Z, Miller GM. (2008) Beta-phenylethylamine alters monoamine transporter function via trace amine-associated receptor 1: implication for modulatory roles of trace amines in brain.
J Pharmacol Exp Ther, 325 (2): 617-28.
[PMID:18182557]
65. Xie Z, Miller GM. (2009) Trace amine-associated receptor 1 as a monoaminergic modulator in brain.
Biochem Pharmacol, 78 (9): 1095-104.
[PMID:19482011]
66. Xie Z, Westmoreland SV, Bahn ME, Chen GL, Yang H, Vallender EJ, Yao WD, Madras BK, Miller GM. (2007) Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro.
J Pharmacol Exp Ther, 321 (1): 116-27.
[PMID:17234900]
67. Xie Z, Westmoreland SV, Miller GM. (2008) Modulation of monoamine transporters by common biogenic amines via trace amine-associated receptor 1 and monoamine autoreceptors in human embryonic kidney 293 cells and brain synaptosomes.
J Pharmacol Exp Ther, 325 (2): 629-40.
[PMID:18310473]