
Top ▲

Target not currently curated in GtoImmuPdb
Target id: 928
Nomenclature: SERT
Systematic Nomenclature: SLC6A4
Family: Monoamine transporter subfamily
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 12 | 630 | 17q11.2 | SLC6A4 | solute carrier family 6 member 4 | |
| Mouse | 12 | 630 | 11 46.18 cM | Slc6a4 | solute carrier family 6 (neurotransmitter transporter, serotonin), member 4 | |
| Rat | 12 | 630 | 10q24 | Slc6a4 | solute carrier family 6 member 4 | |
Database Links  |
|
| Specialist databases | |
| Bioparadigms SLC Tables | SLC6A4 (Hs) |
| RESOLUTE | SLC6A4 (Hs) |
| Other databases | |
| Alphafold | P31645 (Hs), Q60857 (Mm), P31652 (Rn) |
| ChEMBL Target | CHEMBL228 (Hs), CHEMBL4642 (Mm), CHEMBL313 (Rn) |
| DrugBank Target | P31645 (Hs) |
| Ensembl Gene | ENSG00000108576 (Hs), ENSMUSG00000020838 (Mm), ENSRNOG00000003476 (Rn) |
| Entrez Gene | 6532 (Hs), 15567 (Mm), 25553 (Rn) |
| Human Protein Atlas | ENSG00000108576 (Hs) |
| KEGG Gene | hsa:6532 (Hs), mmu:15567 (Mm), rno:25553 (Rn) |
| OMIM | 182138 (Hs) |
| Pharos | P31645 (Hs) |
| SynPHARM |
83551 (in complex with escitalopram) 79385 (in complex with escitalopram) 83552 (in complex with paroxetine) 13002 (in complex with paroxetine) |
| UniProtKB | P31645 (Hs), Q60857 (Mm), P31652 (Rn) |
| Wikipedia | SLC6A4 (Hs) |
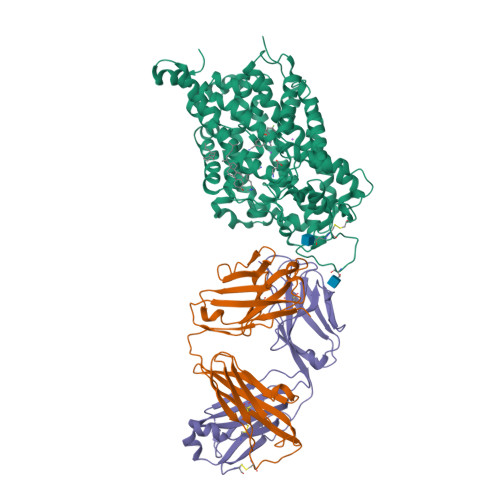
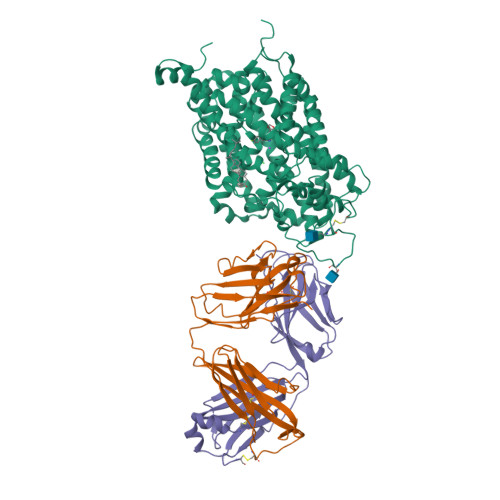
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
Substrates and Reaction Kinetics  |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
Stoichiometry  |
|
| 1 5-HT:1 Na+:1 Cl- (in), + 1 K+ (out) [23] | |
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific inhibitor tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inhibitor Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Although nefazodone is claimed to be an inhibitor of the serotonin and norepinephrine transporters, the dopamine transporter appears to have a similar dissociation constant to the norepinephrine transporter [6]. Occupancy of the allosteric site by escitalopram sterically hinders ligand unbinding from the central site. This allows the drug to negatively regulate (inhibit) SERT activity [7]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||
|
||||||||||
|
||||||||||
1. Altenbach RJ, Black LA, Strakhova MI, Manelli AM, Carr TL, Marsh KC, Wetter JM, Wensink EJ, Hsieh GC, Honore P et al.. (2010) Diaryldiamines with dual inhibition of the histamine H(3) receptor and the norepinephrine transporter and the efficacy of 4-(3-(methylamino)-1-phenylpropyl)-6-(2-(pyrrolidin-1-yl)ethoxy)naphthalen-1-ol in pain. J Med Chem, 53 (21): 7869-73. [PMID:20945906]
2. Arunotayanun W, Dalley JW, Huang XP, Setola V, Treble R, Iversen L, Roth BL, Gibbons S. (2013) An analysis of the synthetic tryptamines AMT and 5-MeO-DALT: emerging 'Novel Psychoactive Drugs'. Bioorg Med Chem Lett, 23 (11): 3411-5. [PMID:23602445]
3. Auerbach SS, DrugMatrix® and ToxFX® Coordinator National Toxicology Program. National Toxicology Program: Dept of Health and Human Services. Accessed on 02/05/2014. Modified on 02/05/2014. DrugMatrix, https://ntp.niehs.nih.gov/drugmatrix/index.html
4. Bang-Andersen B, Ruhland T, Jørgensen M, Smith G, Frederiksen K, Jensen KG, Zhong H, Nielsen SM, Hogg S, Mørk A et al.. (2011) Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem, 54 (9): 3206-21. [PMID:21486038]
5. Ben-Daniel R, Deuther-Conrad W, Scheunemann M, Steinbach J, Brust P, Mishani E. (2008) Carbon-11 labeled indolylpropylamine analog as a new potential PET agent for imaging of the serotonin transporter. Bioorg Med Chem, 16 (12): 6364-70. [PMID:18487050]
6. Carlier PR, Lo MM, Lo PC, Richelson E, Tatsumi M, Reynolds IJ, Sharma TA. (1998) Synthesis of a potent wide-spectrum serotonin-, norepinephrine-, dopamine-reuptake inhibitor (SNDRI) and a species-selective dopamine-reuptake inhibitor based on the gamma-amino alcohol functional group. Bioorg Med Chem Lett, 8 (5): 487-92. [PMID:9871604]
7. Coleman JA, Green EM, Gouaux E. (2016) X-ray structures and mechanism of the human serotonin transporter. Nature, 532 (7599): 334-9. [PMID:27049939]
8. Dawson LA, Watson JM. (2009) Vilazodone: a 5-HT1A receptor agonist/serotonin transporter inhibitor for the treatment of affective disorders. CNS Neurosci Ther, 15 (2): 107-17. [PMID:19499624]
9. Dreyfus N, Myers JK, Badescu VO, de Frutos O, de la Puente ML, Ding C, Filla SA, Fynboe K, Gernert DL, Heinz BA et al.. (2013) Discovery of a potent, dual serotonin and norepinephrine reuptake inhibitor. ACS Med Chem Lett, 4 (6): 560-4. [PMID:24900709]
10. Fish PV, Deur C, Gan X, Greene K, Hoople D, Mackenny M, Para KS, Reeves K, Ryckmans T, Stiff C et al.. (2008) Design and synthesis of morpholine derivatives. SAR for dual serotonin & noradrenaline reuptake inhibition. Bioorg Med Chem Lett, 18 (8): 2562-6. [PMID:18387300]
11. Gengo PJ, Giuliano F, McKenna KE et al.. (2005) Monoaminergic transporter binding and inhibition profile of dapoxetine, a medication for the treatment of premature ejaculation [abstract]. J Urol, 173: Abstract 878.
12. Heinrich T, Böttcher H, Gericke R, Bartoszyk GD, Anzali S, Seyfried CA, Greiner HE, Van Amsterdam C. (2004) Synthesis and structure--activity relationship in a class of indolebutylpiperazines as dual 5-HT(1A) receptor agonists and serotonin reuptake inhibitors. J Med Chem, 47 (19): 4684-92. [PMID:15341484]
13. Inazu M, Takeda H, Ikoshi H, Sugisawa M, Uchida Y, Matsumiya T. (2001) Pharmacological characterization and visualization of the glial serotonin transporter. Neurochem Int, 39 (1): 39-49. [PMID:11311448]
14. Kargbo RB. (2022) Ibogaine and Their Analogs as Therapeutics for Neurological and Psychiatric Disorders. ACS Med Chem Lett, 13 (6): 888-890. [PMID:35707155]
15. Li P, Zhang Q, Robichaud AJ, Lee T, Tomesch J, Yao W, Beard JD, Snyder GL, Zhu H, Peng Y et al.. (2014) Discovery of a tetracyclic quinoxaline derivative as a potent and orally active multifunctional drug candidate for the treatment of neuropsychiatric and neurological disorders. J Med Chem, 57 (6): 2670-82. [PMID:24559051]
16. Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. (1997) Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther, 283 (3): 1305-22. [PMID:9400006]
17. Rotella DP, McFarlane GR, Greenfield A, Grosanu C, Robichaud AJ, Denny RA, Feenstra RW, Núñez-García S, Reinders JH, Neut Mv et al.. (2009) Tetrahydrocarbazole-based serotonin reuptake inhibitor/dopamine D2 partial agonists for the potential treatment of schizophrenia. Bioorg Med Chem Lett, 19 (19): 5552-5. [PMID:19720528]
18. Sabatucci JP, Mahaney PE, Leiter J, Johnston G, Burroughs K, Cosmi S, Zhang Y, Ho D, Deecher DC, Trybulski E. (2010) Heterocyclic cycloalkanol ethylamines as norepinephrine reuptake inhibitors. Bioorg Med Chem Lett, 20 (9): 2809-12. [PMID:20378347]
19. Shobo M, Kondo Y, Yamada H, Mihara T, Yamamoto N, Katsuoka M, Harada K, Ni K, Matsuoka N. (2010) Norzotepine, a major metabolite of zotepine, exerts atypical antipsychotic-like and antidepressant-like actions through its potent inhibition of norepinephrine reuptake. J Pharmacol Exp Ther, 333 (3): 772-81. [PMID:20223878]
20. Snyder GL, Vanover KE, Zhu H, Miller DB, O'Callaghan JP, Tomesch J, Li P, Zhang Q, Krishnan V, Hendrick JP et al.. (2015) Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl.), 232 (3): 605-21. [PMID:25120104]
21. Subbaiah MAM. (2019) Correction to "Triple Reuptake Inhibitors as Potential Therapeutics for Depression and Other Disorders: Design Paradigm and Developmental Challenges". J Med Chem, 62 (21): 10003. [PMID:31617718]
22. Sweetnam PM, Caldwell L, Lancaster J, Bauer Jr C, McMillan B, Kinnier WJ, Price CH. (1993) The role of receptor binding in drug discovery. J Nat Prod, 56 (4): 441-55. [PMID:8496700]
23. Talvenheimo J, Fishkes H, Nelson PJ, Rudnick G. (1983) The serotonin transporter-imipramine "receptor". J Biol Chem, 258 (10): 6115-9. [PMID:6853478]
24. Tatsumi M, Groshan K, Blakely RD, Richelson E. (1997) Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol, 340 (2-3): 249-58. [PMID:9537821]
25. Van Orden LJ, Van Dyke PM, Saito DR, Church TJ, Chang R, Smith JA, Martin WJ, Jaw-Tsai S, Stangeland EL. (2013) A novel class of 3-(phenoxy-phenyl-methyl)-pyrrolidines as potent and balanced norepinephrine and serotonin reuptake inhibitors: synthesis and structure-activity relationships. Bioorg Med Chem Lett, 23 (5): 1456-61. [PMID:23347683]
26. Vickers T, Dyck B, Tamiya J, Zhang M, Jovic F, Grey J, Fleck BA, Aparicio A, Johns M, Jin L et al.. (2008) Studies on a series of milnacipran analogs containing a heteroaromatic group as potent norepinephrine and serotonin transporter inhibitors. Bioorg Med Chem Lett, 18 (11): 3230-5. [PMID:18468895]
27. Xu X, Wei Y, Guo Q, Zhao S, Liu Z, Xiao T, Liu Y, Qiu Y, Hou Y, Zhang G et al.. (2018) Pharmacological Characterization of H05, a Novel Serotonin and Noradrenaline Reuptake Inhibitor with Moderate 5-HT2A Antagonist Activity for the Treatment of Depression. J Pharmacol Exp Ther, 365 (3): 624-635. [PMID:29615471]