|
Comment: Isolated from the leaves of Syagrus orinocensis. The structure of kallstroemin D is shown here without representing the compound's complex stereochemisty. Please see the listed reference for an image of the exact structure.
|
|
2D Structure
|
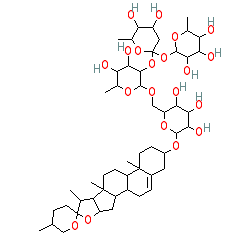
|
Physico-chemical Properties
|
|
|
Hydrogen bond acceptors
|
20
|
|
Hydrogen bond donors
|
10
|
|
Rotatable bonds
|
9
|
|
Topological polar surface area
|
294.6
|
|
Molecular weight
|
1014.54
|
|
XLogP
|
2.88
|
|
No. Lipinski's rules broken
|
2
|
|
SMILES / InChI / InChIKey
|
|
|
Canonical SMILES
|
CC1CCC2(OC1)OC1C(C2C)C2(C(C1)C1CC=C3C(C1CC2)(C)CCC(C3)OC1OC(COC2OC(C)C(C(C2OC2(CC(O)C(C(O2)C)O)OC2OC(C)C(C(C2O)O)O)O)O)C(C(C1O)O)O)C
|
|
Isomeric SMILES
|
CC1CCC2(OC1)OC1C(C2C)C2(C(C1)C1CC=C3C(C1CC2)(C)CCC(C3)OC1OC(COC2OC(C)C(C(C2OC2(CC(O)C(C(O2)C)O)OC2OC(C)C(C(C2O)O)O)O)O)C(C(C1O)O)O)C
|
|
InChI
|
InChI=1S/C51H82O20/c1-21-10-15-50(63-19-21)22(2)34-32(69-50)17-30-28-9-8-26-16-27(11-13-48(26,6)29(28)12-14-49(30,34)7)66-45-42(60)40(58)38(56)33(67-45)20-62-47-44(41(59)37(55)24(4)65-47)70-51(18-31(52)35(53)25(5)68-51)71-46-43(61)39(57)36(54)23(3)64-46/h8,21-25,27-47,52-61H,9-20H2,1-7H3
|
|
InChI Key
|
JXANHBVHGVNOQZ-UHFFFAOYSA-N
|
|
|

