
GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Opioid receptors: Introduction
General
The existence of receptors for opiate drugs was first proposed in 1954 by Beckett and Casy [1] based on their studies of structure-activity relationships for antinociceptive activity in a series of synthetic opiates. These receptors are called 'opioid' since we now know their endogenous ligands are peptides with effects resembling those of opiate drugs. Through structure-activity relationship analysis studies, Portoghese and colleagues suggested as early as 1965 that it may be necessary to propose the existence of more than one opioid receptor type or that multiple modes of interaction of ligands with opioid receptors were possible [39]. High-affinity, stereospecific binding sites for opiate drugs were found in brain in 1973 [38,43-44]. The confirmed presence of specific receptors for opiate alkaloid and related synthetic drugs led to a search for endogenous ligands for these receptors and the discovery of the enkephalins [17], β-endorphin [9], and dynorphins [13].
The first definitive evidence that these receptors did not form a homogeneous population was presented by Martin and colleagues [30] in 1976, in their detailed studies of the actions of several analgesic agents in chronically spinalised dogs, a model permitting discrimination of drug action on several reflex responses. The proposed receptor forms were named after the prototypic drugs used in these studies, i.e. the μ (mu, for morphine) receptor and the κ (kappa, for ketocyclazocine) receptor. Pharmacological analysis of opioid peptide effects in guinea-pig ileum and mouse vas deferens led to the discovery of a third opioid receptor named the δ (delta, for deferens) receptor [26]. The three opioid receptors, μ, δ and κ have been cloned and the recombinant receptors shown to have binding and functional characteristics consistent with their endogenous counterparts [12,20-21,41].
A search for related receptors by homology cloning led to the identification of a clone expressing a G protein-coupled receptor, initially called ORL1 [33] or LC132 [4], with significant sequence homology to the previously identified opioid receptors. Since none of the then known endogenous opioid peptides interacted with the ORL1 receptor, a search for an endogenous ligand for this orphan receptor was initiated. Two groups independently isolated and characterized the same novel endogenous peptide from brain extracts. This peptide, named by the two groups nociceptin [31] or orphanin FQ [40], was isolated by its ability to inhibit adenylate cyclase through activation of the ORL1/LC132 receptor expressed in CHO cells. Nociceptin/orphanin FQ (hereafter abbreviated as N/OFQ) is the product of a precursor protein encoded by a novel gene with significant sequence homology to the genes encoding prodynorphin, proenkephalin and pro-opiomelanocortin [34]. These results suggest that a system utilising the peptide N/OFQ has developed, possibly as a result of gene duplication followed by subsequent evolutionary modification. The novel system is structurally related to the endogenous opioid systems, yet is pharmacologically distinct in that there is no significant cross-activation by the opioid peptides, [Met5]- or [Leu5]-enkephalin, or β-endorphin, although dynorphin A has low but measurable affinity for this receptor. The opioid antagonist, naloxone, which binds to μ, δ and κ receptors (with differing affinities), does not have significant affinity for the ORL1/LC132 receptor. These studies indicate that, from a pharmacological perspective, there are two major branches in the opioid peptide-N/OFQ receptor family: the main branch comprising the μ, δ and κ receptors, where naloxone acts as an antagonist; and a second branch with the receptor for N/OFQ which has negligible affinity for naloxone.
Nomenclature of opioid receptors
The nomenclature for the opioid receptors remains controversial. A 1996 review and proposal for a novel nomenclature [11] based on guidelines from NC-IUPHAR has not been widely accepted by the research community. The 1996 proposal recommended replacement of the terms, μ, δ, and κ with the terms OP3, OP1, and OP2, respectively. However, in the three years or more since the publication of this recommendation, almost all papers referring to opioid receptors have continued to use the well-established Greek symbol nomenclature. Many in the field have voiced their concerns that the original Greek symbol nomenclature is now so established that introduction of an alternative nomenclature is both inappropriate and confusing. It is argued that elimination of this well-established terminology will lead to impaired access to, and reduced citation of, the large body of research literature already published on the structure and properties of opioid receptors.
NC-IUPHAR reconvened its opioid receptor subcommittee in late 1999 and charged it with developing revised recommendations for the nomenclature for opioid receptors consistent with the overall guidelines of NC-IUPHAR. This chapter is a preliminary report from the reconstituted subcommittee; all recommendations in this chapter should be considered as interim while the complex issue of nomenclature for this group of receptors is discussed further at national and international meetings. Since discussion continues on a number of complex issues, our recommendations are conservative, emphasising retention of Greek symbol nomenclature. Readers are invited to send their comments to the Chairman for review by the subcommittee. Issues relating to the nomenclature for the receptor for N/OFQ are discussed below.
The opioid receptor family
The very well-defined pattern of structure-activity relationships for agonism and antagonism and the absolute stereochemistry requirements for opiate-like analgesic activity induced Beckett and Casy and others to propose receptors for opiate drugs long before the presence of endogenous ligands for these receptors was established [1,39]. An established pharmacological convention defined actions as 'opioid' when the actions resembled those produced by a prototypic opiate drug such as morphine and were antagonised by naloxone [26]. This convention continues to have wide acceptance. The term "opiate" is now used to describe drugs derived from the opium poppy Papaver somniferum including morphine and codeine, and other semisynthetic drugs derived from these alkaloids or from thebaine. The term is also used to describe the pharmacological properties of a wide range of synthetic drugs with similar pharmacology, but is not used to describe the endogenous peptides with affinity for the receptors activated by morphine and other opiate drugs. These are called "opioid" peptides since they resemble opiates in their affinities for one or more of the OP receptors. Following the convention established by NC-IUPHAR, the receptors themselves are also called "opioid", since they are the primary targets for the endogenous opioid peptides.
Early studies established that the opioid peptide receptors are heterogeneous. Measures of antagonist affinities against various opioid agonists in different systems resulted in unambiguous evidence for heterogeneity of receptor types, and the eventual definition of the μ, δ and κ receptor types [26,30]. This pharmacological classification was later confirmed when three mRNAs for the three receptor types were cloned and characterized [7,12,21,47]. Subsequently, the structures of the gene for the μ receptor [32,45], and later for the δ- and κ- receptors were elucidated.
The OP receptors
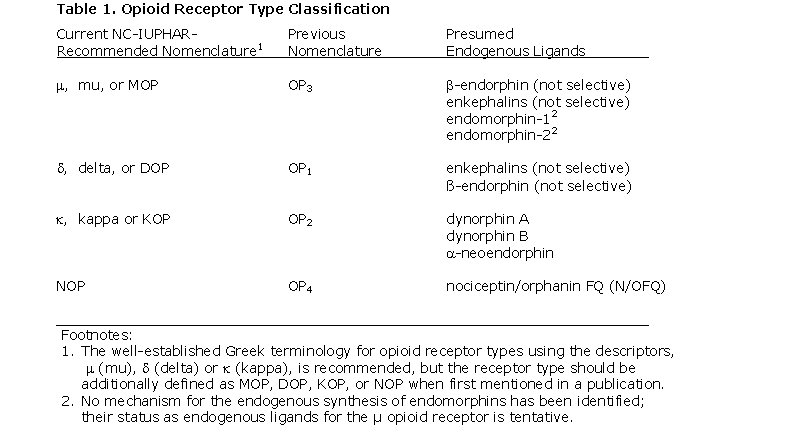
Several abbreviations for the opioid receptor family name have been used; with the simple abbreviation, OR (for opioid receptor), receiving widespread use after the cloning of the receptors in the early 1990s. Unfortunately, this abbreviation is inconsistent with NC-IUPHAR guidelines (the inclusion of 'R' leads too readily to the tautology 'OR receptor' - opioid receptor receptor). Cognisant of these concerns, NC-IUPHAR proposed the abbreviation, OP, as a convenient and unique descriptor for the opioid peptide receptor family [11]. We continue to support this proposal (Table 1).
The NOP receptor
The discovery of the ORL1/LC132 receptor [4,33] has significantly complicated the nomenclature for this family of structurally related receptors. The only known endogenous ligand for this receptor has two names, nociceptin or orphanin FQ, given by the two groups that independently discovered the peptide [31,40]. Neither name is ideal for the peptide and neither name is accepted by all groups publishing in this area. Many authors abbreviate the peptide as N/OFQ; this abbreviation is used here. The structure of the N/OFQ receptor indicates that it has evolved as part of the OP receptor family. Sequence comparisons with μ, κ, and δ receptors, and with other similar G protein-coupled receptors (e.g. of the SOM receptor family), indicate that the N/OFQ receptor is more closely related to OP receptors than to other types of G protein-coupled receptors [3]. Additionally, agonists at N/OFQ receptors induce activation of the same set of transduction pathways activated by μ, κ, and δ receptors (see data tables), and the endogenous ligand, N/OFQ, shares considerable sequence homology with dynorphin A and, to a lesser extent, with the enkephalins. Thus, the N/OFQ receptor and its endogenous ligand are closely related in an evolutionary sense to the μ, κ, and δ receptors.
Despite this evidence of evolutionary and functional homology, the N/OFQ receptor is not an opioid receptor from a pharmacological perspective. The effects of activation of this receptor are not obviously 'opiate-like' with respect to pain perception. The N/OFQ receptor has negligible affinity for naloxone and for most other antagonists at μ, κ or δ receptors. The N/OFQ receptor is, however, expressed in many functional systems in which endogenous opioids play a regulatory role. Although the functions of N/OFQ are not yet fully understood, regulatory functions for N/OFQ parallel to but not identical to those of the endogenous opioid peptides seem very probable. Despite these functional differences, the subcommittee finds the structural relationship between the N/OFQ receptor and μ, δ and κ receptors compelling. Given the evidence, we suggest this receptor be considered a non-opioid branch of the OP family of receptors and propose the abbreviation, NOP (Table 1).
The μ or MOP receptor
The μ receptor was originally defined and characterised pharmacologically by Martin, Kosterlitz and their colleagues on the basis of its high affinity for, and sensitivity to, morphine [26,30]. The endogenous opioids, [Met5]-enkephalin, [Leu5]-enkephalin, extended forms of [Met5]-enkephalin including metorphamide and BAM-18, β-endorphin, and truncated forms of dynorphin (e.g. dynorphin-(1-9) and shorter dynorphin peptides), also have affinities for μ receptors that are consistent with a possible role for each of these peptides as natural ligands for this receptor type, although these endogenous peptides are not selective for μ receptors. Two putative natural ligands, endomorphin-1 and -2, that appear to mediate their effects exclusively through the μ opioid receptor, also have been reported to be present in brain [49] although no gene, precursor protein, or other mechanism for their endogenous synthesis has been identified.
Potent and selective agonists and antagonists for the μ receptor have been developed and these have greatly helped in the characterization of the receptors [27,37]. The μ receptors are distributed throughout the neuraxis. The highest μ receptor densities are found in the thalamus, caudate putamen, neocortex, nucleus accumbens, amygdala, interpeduncular complex, and inferior and superior colliculi [29]. The μ receptors, as well as δ and κ receptors, are also present in the superficial layers of the dorsal horn of spinal cord [2]. A moderate density of μ receptors is found in periaqueductal gray and raphé nuclei [16]. These brain regions have a well-established role in pain and analgesia. Other physiological functions regulated by μ receptors include respiratory and cardiovascular functions, intestinal transit, feeding, mood, thermoregulation, hormone secretion and immune functions [11]. Recent studies have demonstrated that μ receptor distribution in mouse brain is similar to that in rat, with some notable quantitative differences. For example, μ receptors are higher in the hypothalamus in mouse than in rat, but the opposite is true in the hippocampus [22].
The δ or DOP receptor
The δ opioid receptor was defined using the mouse vas deferens preparation and the enkephalins are generally considered the preferred endogenous ligands [26]. Several agonists and antagonists with high affinity and selectivity at δ receptors have been synthesised [41]. The δ receptors are discretely distributed in the central nervous system (CNS), with a prominent gradient of receptor density from high levels in forebrain structures to relatively low levels in most hindbrain regions. The highest densities are found in olfactory bulb, neocortex, caudate putamen, nucleus accumbens, and amygdala [29]. The thalamus and hypothalamus have a moderate density of δ receptors; in more caudal regions the interpeduncular nucleus and pontine nuclei show high binding in rat, but much lower levels in mouse [22]. In the spinal cord, δ receptors are present in dorsal horn where they play a role in mediating the analgesic effects of δ agonists. The functional roles of δ receptors are less clearly established than for μ receptors; in addition to analgesia, δ receptors may have a role in gastrointestinal motility, mood and behaviour as well as in cardiovascular regulation [40].
The κ or KOP receptor
The κ opioid receptor was first proposed on the basis of in vivo studies in dogs with ketocyclazocine and related drugs [30]. Subsequent studies have confirmed the presence of this receptor type in other species including guinea pig, a species that was preferred for many of the early studies on kappa opioid receptors [6].Dynorphins A and B and α-neoendorphin appear to be the endogenous ligands for opioid κ receptors [13], although shorter peptides derived from prodynorphin have comparable affinities at μ and κ receptors. Synthetic compounds (both agonists and antagonists) with selective activity at κ receptors are available (see data tables and [41]). The κ receptors are located predominantly in the cerebral cortex, nucleus accumbens, claustrum and hypothalamus of rat and mouse [22,29], and have been implicated in the regulation of nociception, diuresis, feeding, neuroendocrine and immune system functions [11].
The receptor for N/OFQ: the NOP receptor
The NOP receptor was originally identified by homology cloning as an orphan opioid receptor like clone, ORL1 or LY132 [4,33]. Its endogenous ligand was identified by screening brain extracts for activity at this receptor type in NOP receptor-transfected cells in culture. At present, the only established endogenous ligand for the NOP receptor is N/OFQ itself; none of the previously identified endogenous opioids derived from the proenkephalin, prodynorphin or pro-opiomelanocortin genes has significant affinity or efficacy at this receptor. Natural and synthetic opiate drugs, and the opioid receptor antagonists, naloxone, naltrexone, naltrindole and nor-binaltorphimine are also without significant affinity. Non-peptide drugs related to etorphine and diprenorphine have very low but measurable affinity for the NOP receptor. Recently, two low-molecular weight compounds, J113397 and SB612111, with potent and selective antagonist activity at NOP receptors have been reported [19,52], but these have not yet been widely studied. The synthetic peptides, [NPhe1]nociceptin(1-13)NH2 [5] and UFP-101
NOP receptors are present at relatively high density in selected regions of rat cortex, anterior olfactory nucleus, lateral septum, ventral forebrain, hippocampus, hypothalamus, amygdala, substantia nigra, ventral tegmental area, locus coeruleus, brain stem nuclei and in the dorsal horn of spinal cord [35]. They are also found in immune system cells. This diffuse distribution suggests a role for N/OFQ in many functions including motor and aggressive behaviours, reinforcement and reward, nociception, the stress response, and control of autonomic and immune functions.
Other receptors
In the 1976 studies of Martin et al. [30], which first offered evidence of the heterogeneity of opioid receptors, another receptor type, the σ (sigma; for SKF10047) receptor was also proposed as a form of opioid receptor. Subsequent studies have shown that naloxone does not act as an antagonist at this receptor [28]. A sigma receptor that does not display the seven transmembrane domain (7TM) structure typical of G protein-coupled receptors has recently been cloned [15] and shown to be expressed in the nervous system [42]. There is also evidence for heterogeneity among sigma receptors. However, the sigma receptors defined to date are no longer regarded as members of the OP receptor family.
Other types of opioid receptor have been proposed on the basis of pharmacological properties that appeared incompatible with the well-defined features of μ, δ and κ receptors. These novel opioid receptor types include a β-endorphin-sensitive 'epsilon' (ε) receptor [46], a 'zeta' (ζ) receptor [50] and a high-affinity binding site referred to as the 'lambda' (λ) site [14]. Recently, a zeta receptor gene encoding a protein of 580aa has been identified, based on the high affinity of the expressed protein for [Met5]enkephalin [51]. This receptor does not have any sequence homology with μ, δ and κ opioid receptors, but binding of [Met5]enkephalin to the cloned receptor was inhibited by a high concentration of naltrexone (1μM). No additional binding affinity measures are available. It is proposed that this receptor regulates cell growth; further work is required to confirm the physiological roles of this protein and its relationship to the OP group of receptors. The epsilon and lambda receptors have not been cloned or sequenced to date. Extensive studies conducted to identify additional opioid receptor genes homologous to the μ, δ and κ receptors have met with little success, suggesting that new genes that are structural homologues of the above proposed receptor types are unlikely to be found. The unusual functional properties of the epsilon and lambda receptors might be associated with proteins encoded by non-homologous genes, or with tissue-specific alternative mRNA processing or post-translational modification of the protein products derived from the MOP, DOP, KOP or NOP receptor genes.
There is also speculation that subtypes of the μ, δ and κ receptors may exist (see data tables). Again, there is no evidence suggesting that these subtypes are the products of additional opioid receptor genes. The possible roles of alternative mRNA splicing or post-translational processing remain to be determined. The presence of alternative forms of the μ receptor gene transcript has been established; it is unclear if the function of the receptor proteins expressed by the alternate transcript forms differ significantly but they may differ with respect to rates of internalization and/or recycling [23].
It is also possible that the cellular environment in which a receptor gene is expressed influences the function of the gene product in a tissue- and stimulus-specific manner. Like other GPCRs, OP receptors are subject to agonist-activated phosphorylation by G-protein receptor kinases, and recruitment of a β-arrestin, possibly activarting alternative signaling pathways [25] and leading ultimately to receptor internalization followed by degradation or recycling back to the plasma membrane [24]. OP receptors and associated G proteins may also interact with RGS proteins [48]. The properties of OP receptors in functioning cells are likely to be determined in part by their recent history of activation and association with other membrane proteins. Recent studies have demonstrated that opioid receptors, like other G protein-coupled receptors, can form functional homo- or heterodimers when co-expressed in cells in culture [10]. The functional properties of the heterodimeric forms differ significantly from the properties of either monomer form expressed separately [18]. The potential for the formation of many different forms of OP receptor-protein complexes, possibly varying in prevalence among different cell types, raises the possibility that the each complex may exhibit a unique profile of ligand selectivity and transduction pathway activation. It remains to be determined if the pharmacological evidence for opioid receptor subtypes will eventually be fully or partly explained by the presence of many different forms of OP receptor-membrane protein complexes, including but not limited to OP receptor homo- and heterodimers.
References
1. BECKETT AH, CASY AF. (1954) Synthetic analgesics: stereochemical considerations. J Pharm Pharmacol, 6 (12): 986-1001. [PMID:13212680]
2. Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. (1990) Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res, 521 (1-2): 15-22. [PMID:2169958]
3. Birgül N, Weise C, Kreienkamp HJ, Richter D. (1999) Reverse physiology in drosophila: identification of a novel allatostatin-like neuropeptide and its cognate receptor structurally related to the mammalian somatostatin/galanin/opioid receptor family. EMBO J, 18 (21): 5892-900. [PMID:10545101]
4. Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. (1994) Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett, 347 (2-3): 284-8. [PMID:8034019]
5. Calo' G, Guerrini R, Bigoni R, Rizzi A, Marzola G, Okawa H, Bianchi C, Lambert DG, Salvadori S, Regoli D. (2000) Characterization of [Nphe(1)]nociceptin(1-13)NH(2), a new selective nociceptin receptor antagonist. Br J Pharmacol, 129 (6): 1183-93. [PMID:10725267]
6. Chavkin C, James IF, Goldstein A. (1982) Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science, 215 (4531): 413-5. [PMID:6120570]
7. Chen Y, Mestek A, Liu J, Hurley JA, Yu L. (1993) Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol, 44 (1): 8-12. [PMID:8393525]
8. Connor M, Vaughan CW, Allen RS, Christie MJ. (1999) Nociceptin, Phe1ψ-nociceptin1
9. Cox BM, Goldstein A, Hi CH. (1976) Opioid activity of a peptide, beta-lipotropin-(61-91), derived from beta-lipotropin. Proc Natl Acad Sci USA, 73 (6): 1821-3. [PMID:1064855]
10. Cvejic S, Devi LA. (1997) Dimerization of the delta opioid receptor: implication for a role in receptor internalization. J Biol Chem, 272 (43): 26959-64. [PMID:9341132]
11. Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M. (1996) International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev, 48 (4): 567-92. [PMID:8981566]
12. Evans CJ, Keith Jr DE, Morrison H, Magendzo K, Edwards RH. (1992) Cloning of a delta opioid receptor by functional expression. Science, 258 (5090): 1952-5. [PMID:1335167]
13. Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. (1979) Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci USA, 76 (12): 6666-70. [PMID:230519]
14. Grevel J, Yu V, Sadée W. (1985) Characterization of a labile naloxone binding site (lambda site) in rat brain. J Neurochem, 44 (5): 1647-56. [PMID:2985759]
15. Hanner M, Moebius FF, Flandorfer A, Glossmann H. (1996) Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci USA, 93: 8072-8077. [PMID:8755605]
16. Hawkins KN, Knapp RJ, Gehlert DR, Lui GK, Yamamura MS, Roeske LC, Hruby VJ, Yamamura HI. (1988) Quantitative autoradiography of [3H]CTOP binding to mu opioid receptors in rat brain. Life Sci, 42 (25): 2541-51. [PMID:2898716]
17. Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. (1975) Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature, 258 (5536): 577-80. [PMID:1207728]
18. Jordan BA, Devi LA. (1999) G-protein-coupled receptor heterodimerization modulates receptor function. Nature, 399 (6737): 697-700. [PMID:10385123]
19. Kawamoto H, Ozaki S, Itoh Y, Miyaji M, Arai S, Nakashima H, Kato T, Ohta H, Iwasawa Y. (1999) Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3- hydroxymethyl-4-piperidyl]-3-ethyl-1, 3-dihydro-2H-benzimidazol-2-one (J-113397). J Med Chem, 42 (25): 5061-3. [PMID:10602690]
20. Kieffer BL. (1995) Recent advances in molecular recognition and signal transduction of active peptides: receptors for opioid peptides. Cell Mol Neurobiol, 15 (6): 615-35. [PMID:8719033]
21. Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. (1992) Delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characerization. Proc Natl Acad Sci USA, 89: 12048-12052. [PMID:1334555]
22. Kitchen I, Slowe SJ, Matthes HW, Kieffer B. (1997) Quantitative autoradiographic mapping of mu-, delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain Res, 778 (1): 73-88. [PMID:9462879]
23. Koch T, Schulz S, Schröder H, Wolf R, Raulf E, Höllt V. (1998) Carboxyl-terminal splicing of the rat mu opioid receptor modulates agonist-mediated internalization and receptor resensitization. J Biol Chem, 273 (22): 13652-7. [PMID:9593704]
24. Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Höllt V. (2005) Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol, 67: 280-287. [PMID:15475572]
25. Lefkowitz RJ, Shenoy SK. (2005) Transduction of receptor signals by beta-arrestins. Science, 308: 512-517. [PMID:15845844]
26. Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. (1977) Endogenous opioid peptides: multiple agonists and receptors. Nature, 267 (5611): 495-9. [PMID:195217]
27. Magnan J, Paterson SJ, Tavani A, Kosterlitz HW. (1982) The binding spectrum of narcotic analgesic drugs with different agonist and antagonist properties. Naunyn Schmiedebergs Arch Pharmacol, 319 (3): 197-205. [PMID:6125900]
28. Manallack DT, Beart PM, Gundlach AL. (1986) Psychotomimetic sigma-opiates and PCP. Trends Pharmacol Sci, 7: 448-451.
29. Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. (1987) Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci, 7 (8): 2445-64. [PMID:3039080]
30. Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. (1976) The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther, 197 (3): 517-32. [PMID:945347]
31. Meunier J-C, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B et al.. (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL-1 receptor. Nature, 377: 532-535. [PMID:7566152]
32. Min BH, Augustin LB, Felsheim RF, Fuchs JA, Loh HH. (1994) Genomic structure analysis of promoter sequence of a mouse mu opioid receptor gene. Proc Natl Acad Sci USA, 91 (19): 9081-5. [PMID:8090773]
33. Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. (1994) ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett, 341 (1): 33-8. [PMID:8137918]
34. Mollereau C, Simons MJ, Soularue P, Liners F, Vassart G, Meunier JC, Parmentier M. (1996) Structure, tissue distribution, and chromosomal localization of the prepronociceptin gene. Proc Natl Acad Sci USA, 93 (16): 8666-70. [PMID:8710928]
35. Neal CR, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ. (1999) Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with125I-[14Tyr]-orphanin FQ binding. J Comp Neurol, 412: 563-605. [PMID:10464356]
36. Okuda-Ashitaka E, Minami T, Tachibana S, Yoshihara Y, Nishiuchi Y, Kimura T, Ito S. (1998) Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature, 392 (6673): 286-9. [PMID:9521323]
37. Pelton JT, Kazmierski W, Gulya K, Yamamura HI, Hruby VJ. (1986) Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for mu opioid receptors. J Med Chem, 29 (11): 2370-5. [PMID:2878079]
38. Pert CB, Snyder SH. (1973) Opiate receptor: demonstration in nervous tissue. Science, 179: 1011-1014. [PMID:4687585]
39. Portoghese PS. (1965) A new concept on the mode of interaction of narcotic analgesics with receptors. J Med Chem, 8 (5): 609-16. [PMID:5867942]
40. Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma Jr FJ, Civelli O. (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science, 270 (5237): 792-4. [PMID:7481766]
41. Satoh M, Minami M. (1995) Molecular pharmacology of the opioid receptors. Pharmacol Ther, 68 (3): 343-64. [PMID:8788562]
42. Seth P, Fei YJ, Li HW, Huang W, Leibach FH, Ganapathy V. (1998) Cloning and functional characterization of a sigma receptor from rat brain. J Neurochem, 70 (3): 922-31. [PMID:9489711]
43. Simon EJ, Hiller JM, Edelman I. (1973) Stereospecific binding of the potent narcotic analgesic [3H]etorphine to rat brain homogenate. Proc Natl Acad Sci USA, 70: 1947-1949. [PMID:4516196]
44. Terenius L. (1973) Stereospecific interaction between narcotic analgesics and a synaptic plasma membrane fraction of rat cerebral cortex. Acta Pharmacol Toxicol, 32: 317-320. [PMID:4801733]
45. Wang JB, Johnson PS, Persico AM, Hawkins AL, Griffin CA, Uhl GR. (1994) Human mu opiate receptor. cDNA and genomic clones, pharmacologic characterization and chromosomal assignment. FEBS Lett, 338 (2): 217-22. [PMID:7905839]
46. Wüster M, Schulz R, Herz A. (1979) Specificity of opioids towards the mu-, delta- and epsilon-opiate receptors. Neurosci Lett, 15 (2-3): 193-8. [PMID:231238]
47. Yasuda K, Raynor K, Kong H, Breder CD, Takeda J, Reisine T, Bell GI. (1993) Cloning and functional comparison of kappa and delta opioid receptors from mouse brain. Proc Natl Acad Sci USA, 90 (14): 6736-40. [PMID:8393575]
48. Zachariou V, Georgescu D, Sanchez N, Rahman Z, DiLeone R, Berton O, Neve RL, Sim-Selley LJ, Selley DE, Gold SJ et al.. (2003) Essential role for RGS9 in opiate action. Proc Natl Acad Sci USA, 100 (23): 13656-61. [PMID:14595021]
49. Zadina JE, Hackler L, Ge LJ, Kastin AJ. (1997) A potent and selective endogenous agonist for the mu-opiate receptor. Nature, 386 (6624): 499-502. [PMID:9087409]
50. Zagon IS, Gibo DM, McLaughlin PJ. (1991) Zeta (zeta), a growth-related opioid receptor in developing rat cerebellum: identification and characterization. Brain Res, 551 (1-2): 28-35. [PMID:1655161]
51. Zagon IS, Verderame MF, Allen SS, McLaughlin PJ. (1999) Cloning, sequencing, expression and function of a cDNA encoding a receptor for the opioid growth factor, [Met(5)]enkephalin. Brain Res, 849 (1-2): 147-54. [PMID:10592296]
52. Zaratin PF, Petrone G, Sbacchi M, Garnier M, Fossati C, Petrillo P, Ronzoni S, Giardina GA, Scheideler MA. (2004) Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111). J Pharmacol Exp Ther, 308 (2): 454-61. [PMID:14593080]







