
GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Relaxin family peptide receptors: Introduction
General
The relaxin family peptide (RXFP) receptors are a group of 4 receptors, that mediate the hormonal and neuropeptide actions of the relaxin family peptides relaxin (human gene 2 relaxin or other mammalian equivalents), insulin-like peptide (INSL) 3, relaxin-3 and INSL5 (Table 1). These peptide receptor systems have roles in the cardiovascular system, modulate formation of connective tissue and bone, control aspects of reproduction, and act in the brain to regulate stress responses, anxiety and mood, arousal, spatial and social memory and motivated behaviors, including feeding and drug seeking. Relaxin has vasodilatory, anti-fibrotic, angiogenic, anti-apoptotic and anti-inflammatory properties. The effects of relaxin on tissue remodelling have the potential for far-reaching therapeutic consequences, since fibrosis is a hallmark of all forms of progressive cardiovascular and renal disease and obstructive airway disease (asthma), which collectively contribute to 40-50% of deaths in developed countries. The potent renal [13] and cardiovascular [12] actions of relaxin lead to the first clinical trials in heart failure with relaxin. Phase II trials demonstrated positive renovascular effects and improvements in cardiovascular mortality at 60 and 180-days [56]. Phase IIIa trials demonstrated a significant reduction in mortality at day 180 [54] and importantly, plasma biomarkers of cardiac, renal, and hepatic damage and of decongestion were all significantly improved in serelaxin treated patients [38] consistent with relaxin preventing organ damage. While a subsequent Phase IIIb study did not meet long-term primary endpoints [39], serelaxin treated patients again demonstrated biomarker profiles consistent with positive renovascular effects and prevention of organ damage [55]. Importantly, metanalysis of all the serelaxin trial data suggests treatment of >5000 human patients with relaxin demonstrated no serious adverse effects while producing highly significant reductions in worsening heart failure and markers of renal function, but concluded that a 48 hr short-term treatment of AHF patients may not influence long-term outcomes [55]. Due to these promising results, and the considerable potential of relaxin as an anti-fibrotic, numerous pharmaceutical companies are developing chronic RXFP1 targeting therapies [15,53].
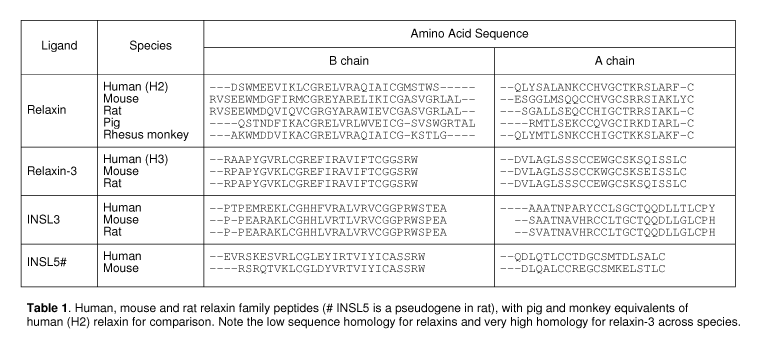
Relaxin was first identified in 1926 as a substance influencing the reproductive tract and was later demonstrated to be a peptide hormone with a two-chain structure similar to insulin. Subsequently several new members of this peptide family were identified, either by differential cloning (insulin-like peptide 3/4 (INSL3, INSL4) or by screening of the EST (INSL5, INSL6) and genomic (relaxin-3) databases. Relaxin-3 is a neuropeptide predominantly expressed in neurons of the nucleus incertus, which project widely to forebrain regions such as the hypothalamus, hippocampus and septum, and other limbic areas [34]. Rodent studies suggest that relaxin-3 has roles in arousal, feeding, stress responses, anxiety and addiction, and attention and memory [41]. The function and cognate receptors of the more recently evolved peptides, INSL4 and INSL6 are currently unknown.
The receptors for relaxin, relaxin-3, INSL3 and INSL5, RXFP1, RXFP2, RXFP3 and RXFP4 have all be cloned and sequenced. Based on the hypothesized coevolution of peptide ligands and their receptors, there was a school of thought that believed the receptors for relaxin and INSL3 were likely to be related to the known insulin receptors and as such be tyrosine kinases. However there was also clear evidence that relaxin caused increases in cAMP in reproductive tissues and cell lines. It is now known, that in spite of their structural similarity, relaxin and insulin family peptides act through independent signalling pathways: the relaxin group activate GPCRs, whereas the insulin group activate tyrosine kinases [25]. Relaxin and INSL3 receptors are a subgroup (type C) of the family of leucine-rich repeat-containing guanine nucleotide binding (G protein)-coupled receptors or LGRs, that include the receptors for the glycoprotein hormones FSH, LH, and TSH and the R-spondins. By using inferences from similar phenotypic expression following mutation and inactivation of INSL3 and a transgenic insertional mutation in mouse chromosome 5, an orphan LGR designated either Great (G protein-coupled receptor affecting testis descent) or LGR8 was postulated to be the INSL3 receptor [42] and this is now known as RXFP2. The discovery that the orphan receptor LGR7 is the relaxin receptor, now RXFP1, was largely attributable to the pursuit of a hunch raised by the combination of the similarity of the structure of RXFP1 to RXFP2 and the similarity of the structure of relaxin to INSL3 [26]. RXFP1 and RXFP2, which in humans are 757 and 737 amino acids in length, respectively, share about 60% amino acid sequence identity and contain 10 leucine-rich repeats in their large N-terminal extracellular domain [5]. Two additional orphan G protein-coupled receptors designated GPCR135 (RXFP3) and GPCR142 (RXFP4) were subsequently identified as receptors for relaxin-3 [31-32]. Unlike RXFP1 and RXFP2, RXFP3 and RXFP4 have short N-terminal extracellular domains, and contain only 469 and 374 amino acid residues, respectively. Cells transfected with RXFP3 [36] were used to identify relaxin-3 as a ligand in porcine brain extracts [32]. The other related receptor, RXFP4, also binds relaxin-3 [32], but on the basis of more recent evidence based on co-localisation of receptor and its cognate ligand it is now clear that it is the receptor for INSL5 [33]. Thus the relaxin family peptides, relaxin, INSL3, relaxin-3 and INSL5 have now been identified as the cognate ligands for the relaxin family peptide (RXFP) receptors 1-4 [4-5,22] (previously known as LGR7, LGR8, GPCR135 and GPCR142).
RXFP1 and RXFP2
RXFP1 has a widespread tissue distribution being found in female and male reproductive tissues, the brain and numerous other non-reproductive tissues such as the kidney, heart and lung. Female reproductive tissues that respond to relaxin include: pubic symphysis, cervix, uterus, nipples and mammary glands, although the relative importance of the functions in these tissues varies with species. Relaxin is also produced in the male reproductive tract, is present in semen and has been suggested to increase sperm motility and penetration into oocytes [7]. There is increasing evidence that relaxin has important roles in the cardiovascular adaptive changes associated with pregnancy. These include increases in plasma volume, cardiac output and heart rate, together with decreased blood pressure and vascular resistance. In brain, RXFP1 is localised to discrete regions of the olfactory system, neocortex, hypothalamus, hippocampus, thalamus, amygdala, midbrain, and medulla of the male and female rat [5,35]. In addition to the specific roles of relaxin already described, it has more general physiological roles. Relaxin inhibits collagen biosynthesis and promotes collagen breakdown in reproductive tissues, but also has similar effects in non-reproductive tissues, which has led to the suggestion that relaxin would be an effective treatment for fibrotic diseases [40,44-45]. It was recently discovered that the anti-fibrotic actions of relaxin are dependent on the presence of the angiotensin AT2 receptor, can be blocked by AT2 antagonists, and are not observed in AT2 knockout mice. Interestingly, the effects of relaxin are also blocked by angiotensin AT1 antagonists suggesting that RXFP1, AT1 and AT2 form complexes that may represent the functional correlate of these findings [10].
RXFP2 is expressed in rat ovary, testis and gubernaculum [7]. In the human, RXFP2 mRNA is found in the uterus and testis in Leydig cells, spermatocytes, spermatids and in the epididymal epithelium [2]. The identical cryptorchid phenotypes of the INSL3 and RXFP2 knockout mice demonstrated that INSL3/RXFP2 is essential for testis descent in rodents. There is less known about the role of INSL3/RXFP2 in adults, however there is evidence that INSL3/RXFP2 is involved in supporting germ cell function in the testis and ovary [28], probably interacting with RXFP2 receptors directly on the germ cells themselves [2]. More recently INSL3 has been shown to be a circulating hormone in women with levels correlating with the number of ovarian antral follicles [1,20] and elevated levels being associated with polycystic ovary syndrome [43]. Furthermore, a recent study in cows demonstrated that RXFP2 is expressed on thecal cells and INSL3 has a positive autoregulatory role in maintaining thecal androgen production that is essential for normal ovarian follicle development [19]. Deficits in INSL3/RXFP2 signaling are also correlated with reduced bone mass [16-17] and RXFP2 mutations may be linked with osteoporosis in men [18], suggesting that INSL3/RXFP2 has a role in bone physiology. RXFP2 is also present in a topographical distribution in the rat brain [50], associated with motor and limbic circuits.
RXFP1 and RXFP2 contain a large extracellular domain (ECD) with a leucine-rich repeat (LRR) domain and linker region connected to a low-density lipoprotein Class A (LDLa) module. The LDLa module is essential for the activation of both RXFP1 and RXFP2 by their ligands [48]. However, the mechanism by which relaxin and INSL3 bind and activate RXFP1 and RXFP2, respectively, are slightly different. INSL3 binding to the RXFP2 LRRs [49] mediates reorientation of the LDLa module by the linker region to activate the RXFP2 transmembrane domains in conjunction with the INSL3 A-chain [8]. In contrast, relaxin binds with high affinity to both the RXFP1 LRRs [9] and the linker [51] and this binding stabilizes a helical conformation of the linker that positions residues of the linker or LDLa + linker to bind the TMD and activate RXFP1 [23,30]. A small molecule RXFP1 agonist ML290 [57] that binds in the transmembrane domain and a relaxin peptidomimetic [24] that binds to the LRRs are biased agonists with distinct signalling profiles.
RXFP1 activates adenylyl cyclase, guanylyl cyclase, PKA, PKC, PI-3-kinase, p38MAPK and ERK1/2 and also interacts with the glucocorticoid receptor. Longer term exposure of RXFP1 to relaxin causes changes in the expression of a number of genes including nNOS, VEGF, ETB receptor, MMP-2 and MMP-9 [11,14,37,46]. RXFP1 activation of adenylate cyclase is complex, involves interaction of the receptor with at least three G-proteins, Gαs, GαoB and Gαi3, and results in a biphasic pattern of cAMP accumulation [21,46-47]. RXFP2 activates adenylate cyclase in recombinant systems but some physiological responses are sensitive to pertussis toxin. It is now becoming clear that the interaction of RXFP2 with adenylate cyclase involves a subset of G proteins utilised by RXFP1 and that the differences may explain the different patterns of cAMP accumulation observed in vivo [21]).
RXFP3 and RXFP4
RXFP3 is predominantly expressed in the brain, whereas RXFP4 is present in brain, kidney, testis, thymus, placenta, prostate, salivary gland, thyroid and colon [7,32]. RXFP3 is present in many areas of the brain, in specific nuclei and neuron types, including in the olfactory bulb, cerebral cortex, septum, hippocampus, amygdala, hypothalamus, thalamus, midbrain, and brainstem [33]. Recent neurophysiological studies of hypothalamic oxytocin- and arginine-vasopressin-synthesizing magnocellular neurosecretory neurons indicate that RXFP3 activation inhibits these cells in male and female rats, and that this inhibition depends on an M-like potassium conductance [27].
In contrast to the high affinity interactions between relaxin and RXFP1, and INSL3 and RXFP2, relaxin-3 has a lower affinity for RXFP1 than relaxin [6,52]. Relaxin-3 is the cognate ligand for RXFP3, but it can also activate RXFP4 [31-32]; although this interaction is unlikely to have physiological significance because of a mismatch of sites of synthesis, storage and release. The receptors differ structurally and functionally from RXFP1 and RXFP2. They have relatively short N-terminal extracellular domains, and couple predominantly to Gαi/o. Studies with native relaxin-3 purified from brain extracts and recombinant human relaxin-3 indicated that this peptide potently stimulates GTPγS binding and inhibits cAMP accumulation in cells expressing RXFP3 or RXFP4. Based on the patterns of expression of ligand and receptor, relaxin-3 is recognised as the cognate ligand for RXFP3 and INSL5 is the cognate ligand for RXFP4 [33]. Studies of RXFP3 signalling reveal that in addition to inhibition of cAMP production, RXFP3 also activates p38MAPK, JNK1/2 and ERK1/2 when activated by relaxin-3 and that relaxin causes biased signaling [29]. RXFP4 when activated by INSL5 not only inhibits cAMP production, but also causes phosphorylation of ERK1/2, p38MAPK, Akt and S6 ribosomal protein. Signalling is mediated principally through Gi/o proteins and the receptor interacts with GRK2, β-arrestins and readily internalizes [3].
References
1. Anand-Ivell R, Tremellen K, Dai Y, Heng K, Yoshida M, Knight PG, Hale GE, Ivell R. (2013) Circulating insulin-like factor 3 (INSL3) in healthy and infertile women. Hum Reprod, 28 (11): 3093-102. [PMID:24014601]
2. Anand-Ivell RJ, Relan V, Balvers M, Coiffec-Dorval I, Fritsch M, Bathgate RA, Ivell R. (2006) Expression of the Insulin-Like Peptide 3 (INSL3) Hormone-Receptor (LGR8) System in the Testis. Biol Reprod, 74: 945-953. [PMID:16467492]
3. Ang SY, Hutchinson DS, Patil N, Evans BA, Bathgate RAD, Halls ML, Hossain MA, Summers RJ, Kocan M. (2017) Signal transduction pathways activated by insulin-like peptide 5 at the relaxin family peptide RXFP4 receptor. Br J Pharmacol, 174 (10): 1077-1089. [PMID:27243554]
4. Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. (2013) Relaxin family peptides and their receptors. Physiol Rev, 93 (1): 405-80. [PMID:23303914]
5. Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. (2006) International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol Rev, 58 (1): 7-31. [PMID:16507880]
6. Bathgate RA, Samuel CS, Burazin TC, Layfield S, Claasz AA, Reytomas IG, Dawson NF, Zhao C, Bond C, Summers RJ et al.. (2002) Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. Novel members of the relaxin peptide family. J Biol Chem, 277 (2): 1148-57. [PMID:11689565]
7. Bathgate RAD, Hsueh AJW, Sherwood OD. (2005) Physiology and molecular biology of the relaxin peptide family. In Physiology of Reproduction Edited by Knobil E, Neill JD (Elsevier) 679-968. [ISBN:0125154003]
8. Bruell S, Sethi A, Smith N, Scott DJ, Hossain MA, Wu QP, Guo ZY, Petrie EJ, Gooley PR, Bathgate RAD. (2017) Distinct activation modes of the Relaxin Family Peptide Receptor 2 in response to insulin-like peptide 3 and relaxin. Sci Rep, 7 (1): 3294. [PMID:28607406]
9. Büllesbach EE, Schwabe C. (2005) The trap-like relaxin-binding site of the leucine-rich G-protein-coupled receptor 7. J Biol Chem, 280 (14): 14051-6. [PMID:15695505]
10. Chow BS, Kocan M, Bosnyak S, Sarwar M, Wigg B, Jones ES, Widdop RE, Summers RJ, Bathgate RA, Hewitson TD et al.. (2014) Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int, 86 (1): 75-85. [PMID:24429402]
11. Conrad KP. (2010) Unveiling the vasodilatory actions and mechanisms of relaxin. Hypertension, 56 (1): 2-9. [PMID:20497994]
12. Conrad KP, Debrah DO, Novak J, Danielson LA, Shroff SG. (2004) Relaxin modifies systemic arterial resistance and compliance in conscious, nonpregnant rats. Endocrinology, 145 (7): 3289-96. [PMID:15198972]
13. Danielson LA, Kercher LJ, Conrad KP. (2000) Impact of gender and endothelin on renal vasodilation and hyperfiltration induced by relaxin in conscious rats. Am J Physiol Regul Integr Comp Physiol, 279 (4): R1298-304. [PMID:11003996]
14. Dschietzig T, Bartsch C, Richter C, Laule M, Baumann G, Stangl K. (2003) Relaxin, a pregnancy hormone, is a functional endothelin-1 antagonist: attenuation of endothelin-1-mediated vasoconstriction by stimulation of endothelin type-B receptor expression via ERK-1/2 and nuclear factor-kappaB. Circ Res, 92 (1): 32-40. [PMID:12522118]
15. Dschietzig TB. (2019) Relaxin-2 for heart failure with preserved ejection fraction (HFpEF): Rationale for future clinical trials. Mol Cell Endocrinol, 487: 54-58. [PMID:30659842]
16. Ferlin A, Pepe A, Gianesello L, Garolla A, Feng S, Facciolli A, Morello R, Agoulnik AI, Foresta C. (2009) New roles for INSL3 in adults. Ann N Y Acad Sci, 1160: 215-8. [PMID:19416191]
17. Ferlin A, Pepe A, Gianesello L, Garolla A, Feng S, Giannini S, Zaccolo M, Facciolli A, Morello R, Agoulnik AI et al.. (2008) Mutations in the insulin-like factor 3 receptor are associated with osteoporosis. J Bone Miner Res, 23 (5): 683-93. [PMID:18433302]
18. Ferlin A, Selice R, Carraro U, Foresta C. (2013) Testicular function and bone metabolism--beyond testosterone. Nat Rev Endocrinol, 9 (9): 548-54. [PMID:23856820]
19. Glister C, Satchell L, Bathgate RA, Wade JD, Dai Y, Ivell R, Anand-Ivell R, Rodgers RJ, Knight PG. (2013) Functional link between bone morphogenetic proteins and insulin-like peptide 3 signaling in modulating ovarian androgen production. Proc Natl Acad Sci USA, 110 (15): E1426-35. [PMID:23530236]
20. Hagen CP, Mieritz MG, Nielsen JE, Anand-Ivell R, Ivell R, Juul A. (2015) Longitudinal assessment of circulating insulin-like peptide 3 levels in healthy peripubertal girls. Fertil Steril, 103 (3): 780-6.e1. [PMID:25516081]
21. Halls ML, Bathgate RA, Summers RJ. (2006) Relaxin family peptide receptors RXFP1 and RXFP2 modulate cAMP signaling by distinct mechanisms. Mol Pharmacol, 70 (1): 214-26. [PMID:16569707]
22. Halls ML, Bathgate RA, Sutton SW, Dschietzig TB, Summers RJ. (2015) International Union of Basic and Clinical Pharmacology. XCV. Recent advances in the understanding of the pharmacology and biological roles of relaxin family peptide receptors 1-4, the receptors for relaxin family peptides. Pharmacol Rev, 67 (2): 389-440. [PMID:25761609]
23. Hopkins EJ, Layfield S, Ferraro T, Bathgate RA, Gooley PR. (2007) The NMR solution structure of the relaxin (RXFP1) receptor lipoprotein receptor class A module and identification of key residues in the N-terminal region of the module that mediate receptor activation. J Biol Chem, 282 (6): 4172-84. [PMID:17148455]
24. Hossain MA, Kocan M, Yao ST, Royce SG, Nair VB, Siwek C, Patil NA, Harrison IP, Rosengren KJ, Selemidis S et al.. (2016) A single-chain derivative of the relaxin hormone is a functionally selective agonist of the G protein-coupled receptor, RXFP1. Chem Sci, 7 (6): 3805-3819. [PMID:30155023]
25. Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, van der Spek PJ, van Duin M, Hsueh AJ. (2000) The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol, 14 (8): 1257-71. [PMID:10935549]
26. Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ. (2002) Activation of orphan receptors by the hormone relaxin. Science, 295 (5555): 671-4. [PMID:11809971]
27. Kania A, Szlaga A, Sambak P, Gugula A, Blasiak E, Micioni Di Bonaventura MV, Hossain MA, Cifani C, Hess G, Gundlach AL et al.. (2020) RLN3/RXFP3 Signaling in the PVN Inhibits Magnocellular Neurons via M-like Current Activation and Contributes to Binge Eating Behavior. J Neurosci, 40 (28): 5362-5375. [PMID:32532885]
28. Kawamura K, Kumagai J, Sudo S, Chun SY, Pisarska M, Morita H, Toppari J, Fu P, Wade JD, Bathgate RA et al.. (2004) Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci USA, 101 (19): 7323-8. [PMID:15123806]
29. Kocan M, Sarwar M, Hossain MA, Wade JD, Summers RJ. (2014) Signalling profiles of H3 relaxin, H2 relaxin and R3(BΔ23-27)R/I5 acting at the relaxin family peptide receptor 3 (RXFP3). Br J Pharmacol, 171 (11): 2827-41. [PMID:24641548]
30. Kong RC, Petrie EJ, Mohanty B, Ling J, Lee JC, Gooley PR, Bathgate RA. (2013) The relaxin receptor (RXFP1) utilizes hydrophobic moieties on a signaling surface of its N-terminal low density lipoprotein class A module to mediate receptor activation. J Biol Chem, 288 (39): 28138-51. [PMID:23926099]
31. Liu C, Chen J, Sutton S, Roland B, Kuei C, Farmer N, Sillard R, Lovenberg TW. (2003) Identification of relaxin-3/INSL7 as a ligand for GPCR142. J Biol Chem, 278 (50): 50765-70. [PMID:14522967]
32. Liu C, Eriste E, Sutton S, Chen J, Roland B, Kuei C, Farmer N, Jörnvall H, Sillard R, Lovenberg TW. (2003) Identification of relaxin-3/INSL7 as an endogenous ligand for the orphan G-protein-coupled receptor GPCR135. J Biol Chem, 278 (50): 50754-64. [PMID:14522968]
33. Liu C, Kuei C, Sutton S, Chen J, Bonaventure P, Wu J, Nepomuceno D, Kamme F, Tran DT, Zhu J et al.. (2005) INSL5 is a high affinity specific agonist for GPCR142 (GPR100). J Biol Chem, 280 (1): 292-300. [PMID:15525639]
34. Ma S, Bonaventure P, Ferraro T, Shen PJ, Burazin TC, Bathgate RA, Liu C, Tregear GW, Sutton SW, Gundlach AL. (2007) Relaxin-3 in GABA projection neurons of nucleus incertus suggests widespread influence on forebrain circuits via G-protein-coupled receptor-135 in the rat. Neuroscience, 144 (1): 165-90. [PMID:17071007]
35. Ma S, Shen PJ, Burazin TC, Tregear GW, Gundlach AL. (2006) Comparative localization of leucine-rich repeat-containing G-protein-coupled receptor-7 (RXFP1) mRNA and [33P]-relaxin binding sites in rat brain: restricted somatic co-expression a clue to relaxin action?. Neuroscience, 141 (1): 329-44. [PMID:16725278]
36. Matsumoto M, Kamohara M, Sugimoto T, Hidaka K, Takasaki J, Saito T, Okada M, Yamaguchi T, Furuichi K. (2000) The novel G-protein coupled receptor SALPR shares sequence similarity with somatostatin and angiotensin receptors. Gene, 248 (1-2): 183-9. [PMID:10806363]
37. McGuane JT, Debrah JE, Sautina L, Jarajapu YP, Novak J, Rubin JP, Grant MB, Segal M, Conrad KP. (2011) Relaxin induces rapid dilation of rodent small renal and human subcutaneous arteries via PI3 kinase and nitric oxide. Endocrinology, 152 (7): 2786-96. [PMID:21558316]
38. Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams Jr KF et al.. (2013) Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol, 61 (2): 196-206. [PMID:23273292]
39. Metra M, Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Voors AA et al.. (2019) Effects of Serelaxin in Patients with Acute Heart Failure. N Engl J Med, 381 (8): 716-726. [PMID:31433919]
40. Mookerjee I, Solly NR, Royce SG, Tregear GW, Samuel CS, Tang ML. (2006) Endogenous relaxin regulates collagen deposition in an animal model of allergic airway disease. Endocrinology, 147 (2): 754-61. [PMID:16254028]
41. Olucha-Bordonau FE, Albert-Gascó H, Ros-Bernal F, Rytova V, Ong-Pålsson EKE, Ma S, Sánchez-Pérez AM, Gundlach AL. (2018) Modulation of forebrain function by nucleus incertus and relaxin-3/RXFP3 signaling. CNS Neurosci Ther, 24 (8): 694-702. [PMID:29722152]
42. Overbeek PA, Gorlov IP, Sutherland RW, Houston JB, Harrison WR, Boettger-Tong HL, Bishop CE, Agoulnik AI. (2001) A transgenic insertion causing cryptorchidism in mice. Genesis, 30 (1): 26-35. [PMID:11353515]
43. Pelusi C, Fanelli F, Pariali M, Zanotti L, Gambineri A, Pasquali R. (2013) Parallel variations of insulin-like peptide 3 (INSL3) and antimüllerian hormone (AMH) in women with the polycystic ovary syndrome according to menstrual cycle pattern. J Clin Endocrinol Metab, 98 (10): E1575-82. [PMID:23928669]
44. Samuel CS. (2005) Relaxin: antifibrotic properties and effects in models of disease. Clin Med Res, 3 (4): 241-9. [PMID:16303890]
45. Samuel CS, Zhao C, Bathgate RA, DU XJ, Summers RJ, Amento EP, Walker LL, McBurnie M, Zhao L, Tregear GW. (2005) The relaxin gene-knockout mouse: a model of progressive fibrosis. Ann N Y Acad Sci, 1041: 173-181. [PMID:15956703]
46. Sarwar M, Samuel CS, Bathgate RA, Stewart DR, Summers RJ. (2015) Serelaxin-mediated signal transduction in human vascular cells: bell-shaped concentration-response curves reflect differential coupling to G proteins. Br J Pharmacol, 172 (4): 1005-19. [PMID:25297987]
47. Sarwar M, Samuel CS, Bathgate RA, Stewart DR, Summers RJ. (2016) Enhanced serelaxin signalling in co-cultures of human primary endothelial and smooth muscle cells. Br J Pharmacol, 173 (3): 484-96. [PMID:26493539]
48. Scott DJ, Layfield S, Yan Y, Sudo S, Hsueh AJ, Tregear GW, Bathgate RA. (2006) Characterization of novel splice variants of LGR7 and LGR8 reveals that receptor signaling is mediated by their unique low density lipoprotein class A modules. J Biol Chem, 281 (46): 34942-54. [PMID:16963451]
49. Scott DJ, Wilkinson TN, Zhang S, Ferraro T, Wade JD, Tregear GW, Bathgate RA. (2007) Defining the LGR8 residues involved in binding insulin-like peptide 3. Mol Endocrinol, 21 (7): 1699-712. [PMID:17473281]
50. Sedaghat K, Shen PJ, Finkelstein DI, Henderson JM, Gundlach AL. (2008) Leucine-rich repeat-containing G-protein-coupled receptor 8 in the rat brain: Enrichment in thalamic neurons and their efferent projections. Neuroscience, 156 (2): 319-33. [PMID:18706979]
51. Sethi A, Bruell S, Patil N, Hossain MA, Scott DJ, Petrie EJ, Bathgate RA, Gooley PR. (2016) The complex binding mode of the peptide hormone H2 relaxin to its receptor RXFP1. Nat Commun, 7: 11344. [PMID:27088579]
52. Sudo S, Kumagai J, Nishi S, Layfield S, Ferraro T, Bathgate RA, Hsueh AJ. (2003) H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J Biol Chem, 278 (10): 7855-62. [PMID:12506116]
53. Sun J, Hao W, Fillmore N, Ma H, Springer D, Yu ZX, Sadowska A, Garcia A, Chen R, Muniz-Medina V et al.. (2019) Human Relaxin-2 Fusion Protein Treatment Prevents and Reverses Isoproterenol-Induced Hypertrophy and Fibrosis in Mouse Heart. J Am Heart Assoc, 8 (24): e013465. [PMID:31818212]
54. Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams Jr KF et al.. (2013) Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet, 381 (9860): 29-39. [PMID:23141816]
55. Teerlink JR, Davison BA, Cotter G, Maggioni AP, Sato N, Chioncel O, Ertl G, Felker GM, Filippatos G, Greenberg BH et al.. (2020) Effects of serelaxin in patients admitted for acute heart failure: a meta-analysis. Eur J Heart Fail, 22 (2): 315-329. [PMID:31886953]
56. Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, Marmor A, Katz A, Grzybowski J, Unemori E et al.. (2009) Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet, 373 (9673): 1429-39. [PMID:19329178]
57. Xiao J, Chen CZ, Huang Z, Agoulnik IU, Ferrer M, Southall N, Hu X, Zheng W, Agoulnik AI, Marugan JJ. (2010) Discovery, optimization, and biological activity of the first potent and selective small-molecule agonist series of human relaxin receptor 1 (RXFP1). Probe Reports from the NIH Molecular Libraries Program,. [PMID:23905199]







