|
|
|
2D Structure
|
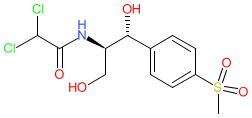
|
Physico-chemical Properties
|
|
|
Hydrogen bond acceptors
|
6
|
|
Hydrogen bond donors
|
3
|
|
Rotatable bonds
|
7
|
|
Topological polar surface area
|
112.08
|
|
Molecular weight
|
355
|
|
XLogP
|
0.31
|
|
No. Lipinski's rules broken
|
0
|
|
SMILES / InChI / InChIKey
|
|
|
Canonical SMILES
|
OC[C@H]([C@@H](c1ccc(cc1)S(=O)(=O)C)O)NC(=O)C(Cl)Cl
|
|
Isomeric SMILES
|
CS(=O)(=O)c1ccc(cc1)[C@H]([C@@H](CO)NC(=O)C(Cl)Cl)O
|
|
InChI
|
InChI=1S/C12H15Cl2NO5S/c1-21(19,20)8-4-2-7(3-5-8)10(17)9(6-16)15-12(18)11(13)14/h2-5,9-11,16-17H,6H2,1H3,(H,15,18)/t9-,10-/m1/s1
|
|
InChI Key
|
OTVAEFIXJLOWRX-NXEZZACHSA-N
|
|
|

