
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 4 | 152 | 9q34.11 | PTGES | prostaglandin E synthase | |
| Mouse | 4 | 153 | 2 21.75 cM | Ptges | prostaglandin E synthase | |
| Rat | - | - | Ptges | prostaglandin E synthase | ||
Previous and Unofficial Names  |
| MGST1-L1 | PIG12 | Prostaglandin E synthase | MGST-IV | MGST1-like 1 | microsomal prostaglandin E synthase-1 | Pges |
Database Links  |
|
| Alphafold | O14684 (Hs), Q9JM51 (Mm) |
| BRENDA | 5.3.99.3 |
| CATH/Gene3D | 1.20.120.550 |
| ChEMBL Target | CHEMBL5658 (Hs), CHEMBL2046261 (Mm) |
| Ensembl Gene | ENSG00000148344 (Hs), ENSMUSG00000050737 (Mm), ENSRNOG00000006320 (Rn) |
| Entrez Gene | 9536 (Hs), 64292 (Mm), 59103 (Rn) |
| Human Protein Atlas | ENSG00000148344 (Hs) |
| KEGG Enzyme | 5.3.99.3 |
| KEGG Gene | hsa:9536 (Hs), mmu:64292 (Mm), rno:59103 (Rn) |
| OMIM | 605172 (Hs) |
| Pharos | O14684 (Hs) |
| SynPHARM | 82842 (in complex with compound 30 [PMID: 15953724]) |
| UniProtKB | O14684 (Hs), Q9JM51 (Mm) |
| Wikipedia | PTGES (Hs) |
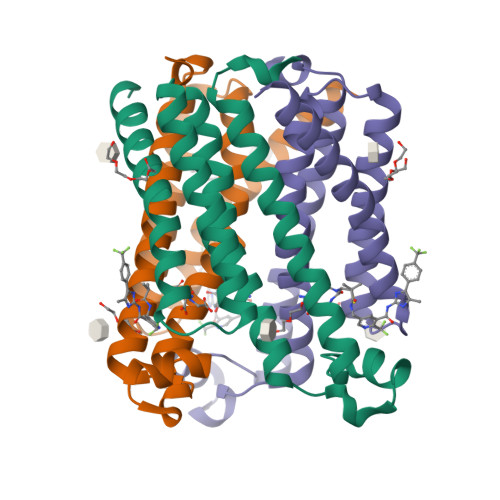
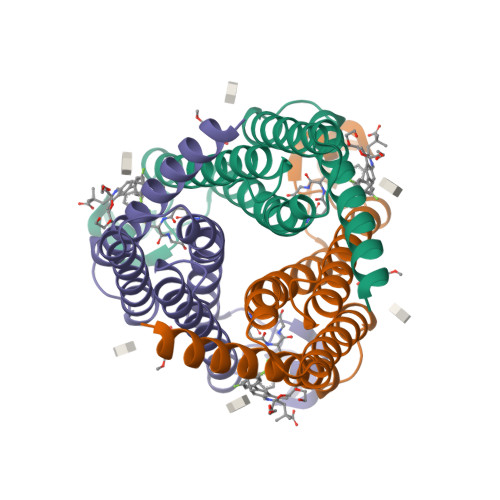
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific inhibitor tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| Inhibition of mPGES-1 is proposed as a potentially safer alternative to COX-2 inhibition for the treatment of pain and inflammation, with a reduced risk of cardiovascular side effects [7]. Inhibition of mPGES-1 in A549 cells and mouse macrophages by compound III [PMID: 24045148] reduces PGE2 production [4]. |
| Immuno Process Associations | ||||||||||||
|
||||||||||||
|
1. Banerjee A, Pawar MY, Patil S, Yadav PS, Kadam PA, Kattige VG, Deshpande DS, Pednekar PV, Pisat MK, Gharat LA. (2014) Development of 2-aryl substituted quinazolin-4(3H)-one, pyrido[4,3-d]pyrimidin-4(3H)-one and pyrido[2,3-d]pyrimidin-4(3H)-one derivatives as microsomal prostaglandin E(2) synthase-1 inhibitors. Bioorg Med Chem Lett, 24 (20): 4838-44. [PMID:25260492]
2. Ergül AG, Jordan PM, Dahlke P, Bal NB, Olğaç A, Uludağ O, Werz O, Çalışkan B, Banoglu E. (2024) Novel Benzimidazole Derivatives as Potent Inhibitors of Microsomal Prostaglandin E2 Synthase 1 for the Potential Treatment of Inflammation, Pain, and Fever. J Med Chem, [Epub ahead of print]. [PMID:39622054]
3. Giroux A, Boulet L, Brideau C, Chau A, Claveau D, Côté B, Ethier D, Frenette R, Gagnon M, Guay J et al.. (2009) Discovery of disubstituted phenanthrene imidazoles as potent, selective and orally active mPGES-1 inhibitors. Bioorg Med Chem Lett, 19 (20): 5837-41. [PMID:19748780]
4. Leclerc P, Idborg H, Spahiu L, Larsson C, Nekhotiaeva N, Wannberg J, Stenberg P, Korotkova M, Jakobsson PJ. (2013) Characterization of a human and murine mPGES-1 inhibitor and comparison to mPGES-1 genetic deletion in mouse models of inflammation. Prostaglandins Other Lipid Mediat, 107: 26-34. [PMID:24045148]
5. Luz JG, Antonysamy S, Kuklish SL, Condon B, Lee MR, Allison D, Yu XP, Chandrasekhar S, Backer R, Zhang A et al.. (2015) Crystal Structures of mPGES-1 Inhibitor Complexes Form a Basis for the Rational Design of Potent Analgesic and Anti-Inflammatory Therapeutics. J Med Chem, 58 (11): 4727-37. [PMID:25961169]
6. Otsu H. (2015) Heterocyclic derivative and pharmaceutical drug. Patent number: US9216968B2. Assignee: Nippon Shinyaku Co Ltd. Priority date: 17/08/2012. Publication date: 22/12/2015.
7. Ozen G, Gomez I, Daci A, Deschildre C, Boubaya L, Teskin O, Uydeş-Doğan BS, Jakobsson PJ, Longrois D, Topal G et al.. (2017) Inhibition of microsomal PGE synthase-1 reduces human vascular tone by increasing PGI2 : a safer alternative to COX-2 inhibition. Br J Pharmacol, 174 (22): 4087-4098. [PMID:28675448]
8. Priepke H, Doods H, Kuelzer R, Pfau R, Stenkamp D, Pelcman B, Roenn R. (2012) New compounds. Patent number: WO2012022793A1. Assignee: Boehringer Ingelheim International Gmbh. Priority date: 20/08/2010. Publication date: 23/02/2012.
9. Riendeau D, Aspiotis R, Ethier D, Gareau Y, Grimm EL, Guay J, Guiral S, Juteau H, Mancini JA, Méthot N et al.. (2005) Inhibitors of the inducible microsomal prostaglandin E2 synthase (mPGES-1) derived from MK-886. Bioorg Med Chem Lett, 15 (14): 3352-5. [PMID:15953724]
10. Schiffler MA, Antonysamy S, Bhattachar SN, Campanale KM, Chandrasekhar S, Condon B, Desai PV, Fisher MJ, Groshong C, Harvey A et al.. (2016) Discovery and Characterization of 2-Acylaminoimidazole Microsomal Prostaglandin E Synthase-1 Inhibitors. J Med Chem, 59 (1): 194-205. [PMID:26653180]
11. Shin JH, Lee YA, Lee JK, Lee YB, Cho W, Im DS, Lee JH, Yun BS, Springer JE, Gwag BJ. (2012) Concurrent blockade of free radical and microsomal prostaglandin E synthase-1-mediated PGE2 production improves safety and efficacy in a mouse model of amyotrophic lateral sclerosis. J Neurochem, 122 (5): 952-61. [PMID:22537108]
12. Shiro T, Kakiguchi K, Takahashi H, Nagata H, Tobe M. (2013) 7-Phenyl-imidazoquinolin-4(5H)-one derivatives as selective and orally available mPGES-1 inhibitors. Bioorg Med Chem, 21 (11): 2868-78. [PMID:23623673]
13. Tandon M, Sant S, Khairatkar-Joshi N, Gudi G, Menon VCA, Talluri R. (2019) Mpges-1 inhibitor for the treatment of osteoarthritis pain. Patent number: WO2013186692A1. Assignee: Glenmark Pharmaceuticals. Priority date: 20/09/2017. Publication date: 28/02/2019.
14. Wannberg J, Alterman M, Malm J, Stenberg P, Westman J, Wallberg H. (2011) Microsomal prostaglandin e synthase-1 (mpges1) inhibitors. Patent number: WO2011023812. Assignee: Novasaid Ab. Priority date: 27/08/2009. Publication date: 03/03/2011.