
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 370 | 20q13.33 | OPRL1 | opioid related nociceptin receptor 1 | 68 |
| Mouse | 7 | 367 | 2 103.74 cM | Oprl1 | opioid receptor-like 1 | 77 |
| Rat | 7 | 367 | 3q43 | Oprl1 | opioid related nociceptin receptor 1 | 9,93 |
Previous and Unofficial Names  |
| N/OFQ receptor | OP4 | KOR-3 | NOCIR | kappa3-related opioid receptor | MOR-C | nociceptin receptor ORL1 | XOR1 | NOP-r | nociceptin/orphanin FQ receptor | NOPr |
Database Links  |
|
| Specialist databases | |
| GPCRdb | oprx_human (Hs), oprx_mouse (Mm), oprx_rat (Rn) |
| Other databases | |
| Alphafold | P41146 (Hs), P35377 (Mm), P35370 (Rn) |
| ChEMBL Target | CHEMBL2014 (Hs), CHEMBL3621 (Mm), CHEMBL4503 (Rn) |
| Ensembl Gene | ENSG00000125510 (Hs), ENSMUSG00000027584 (Mm), ENSRNOG00000016768 (Rn) |
| Entrez Gene | 4987 (Hs), 18389 (Mm), 29256 (Rn) |
| Human Protein Atlas | ENSG00000125510 (Hs) |
| KEGG Gene | hsa:4987 (Hs), mmu:18389 (Mm), rno:29256 (Rn) |
| OMIM | 602548 (Hs) |
| Pharos | P41146 (Hs) |
| RefSeq Nucleotide | NM_000913 (Hs), NM_011012 (Mm), NM_031569 (Rn) |
| RefSeq Protein | NP_000904 (Hs), NP_035142 (Mm), NP_113757 (Rn) |
| SynPHARM |
3979 (in complex with SB 612111) 82972 (in complex with SB 612111) |
| UniProtKB | P41146 (Hs), P35377 (Mm), P35370 (Rn) |
| Wikipedia | OPRL1 (Hs) |
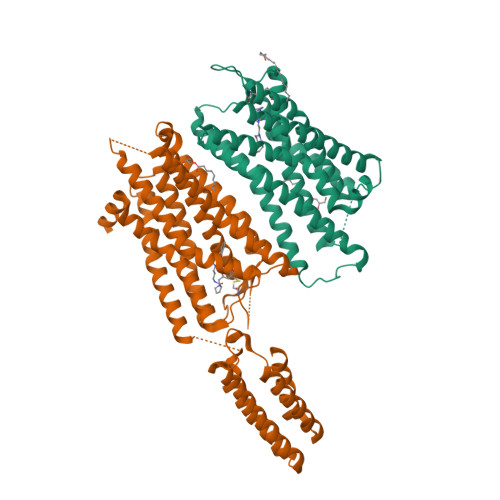
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
Natural/Endogenous Ligands  |
| nociceptin/orphanin FQ {Sp: Human, Mouse, Rat} |
| Principal endogenous agonists |
| nociceptin/orphanin FQ (PNOC, Q13519) [1,8,75] |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific agonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The above affinities are based on the use of radiolabelled N/OFQ to bind to membrane preparations from CHO cells containing the NOP receptors. This represents a high affinity binding conformation in the absence of Na+ and GTP, and low affinity values are not available. The Ki in intact cells, or in the presence of Na+ and GTP analogues can be different and multiple affinity sites have been observed. Discrimination of full or partial agonism is very dependent on the level of receptor expression and on the assay used to monitor agonist effects. For instance [Phe1ψ(CH2-NH)Gly2]NC(1-13)NH2, the first "antagonist" reported [33], has antagonist activity in the guinea pig ileum and mouse vas deferens, but partial agonist activity in transfected cells, and full agonist activity in vivo. The identification of agonist activity in the table is largely based on the ability to stimulate GTPγS binding in cell lines expressing cloned human NOP receptors and in some instances smooth muscle bioassays such as mouse or rat vas deferens. Agents giving 85% or greater stimulation than that given by N/OFQ have been characterized as Full Agonists [24,59]. Results may be different in brain membranes. In vivo and smooth muscle bioassay results may also be different and depend upon the assay performed. Biased Agonists There are have been two publications discussing biased agonism for the NOP receptor [16,52]. In each case compounds with less than 100% efficacy with respect to G protein coupling appeared to be ineffective for β-arrestin coupling. Furthermore, potencies of agonists for the NOP receptor/β-arrestin interaction were systematically lower that those measured for the NOP receptor/G protein interaction in both publications. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The above affinities ase based on binding to receptors in membrane preparations and represent a rough average from, in some cases, multiple studies. So far, no inverse agonists have been reported for the NOP receptor. More details about the pharmacological profile of the NOP receptors and the chemistry of NOP ligands can be found in review articles [10,12,65,99]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulator Comments | ||
| Although no small molecules are considered direct allosteric regulators of the NOP receptor, a number of proteins such as G protein-coupled receptor kinases, β-arrestins and G proteins clearly regulate receptor functions. Furthermore, sodium and guanyl nucleotides can modify the functional NOP complex and G protein interaction. Finally, other G protein-coupled receptors (i.e. the μ opioid receptor [92]) appear to be able to form heterodimers with NOP receptors, potentailly modifying the receptor activity. |
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
| Gi/Go family |
Adenylyl cyclase inhibition Potassium channel Calcium channel Other - See Comments |
| Comments: NOP receptors have been shown to activate MAP kinase and phospholipase C/[Ca2+] [35]. | |
| References: 20,24,63,78,90 | |
Secondary Transduction Mechanisms  |
|
| Transducer | Effector/Response |
| Gi/Go family | Adenylyl cyclase inhibition |
| References: | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
| Tissue Distribution Comments | ||||||||
| NOP receptors are located both pre- and post-synaptically in various areas of the CNS. Brain: cingulate, retrosplenial, perirhinal, insular and occipital cortex, anterior and posteromedial cortical amygdaloid nuclei, basolateral amygdaloid nucleus, amygdaloid complex, posterior hippocampus, dorsal endopiriform, central medial thalamic, paraventricular, rhomboid thalamic, suprachiasmatic, ventromedial hypothalamic nuclei, mammillary complex, superficial gray layer of the superior colliculus, locus coeruleus, dorsal raphe nucleus > prefrontal, fronto-parietal, temporal, piriform cortex, dentate gyrus, anterior olfactory nucleus, olfactory tubercle, shell of nucleus accumbens, claustrum, lateral septum, laterodorsal thalamic, medial habenular, subthalamic, reuniens thalamic nuclei, subiculum, periaqueductal grey matter and pons > anterior and medial hippocampus, olfactory bulb, caudate putamen, the core of the nucleus accumbens, medial septum, ventrolateral, ventroposterolateral and mediodorsal thalamic nuclei, lateral and medial geniculate nuclei, hypothalamic area, substantia nigra, ventral tegmentum area and interpedoncular nucleus. Like the μ opioid receptor, the NOP receptor shows very dense binding in many caudal and rostral regions, but with a notably distinct binding profile. The distinction of labelling in comparison to the classical opioid receptors is most evident in the caudate putamen, where NOP receptors are relatively low. In contrast, structures such as the suprachiasmatic nucleus have an abundant expression of NOP receptors. Studies of the distribution of NOP receptors in humans have also been limited to autoradiography and in situ hybridisation analysis. NOP receptors in the CNS appear to have a similar distribution in rat and human. It should be noted that NOP receptors appear far sooner and in larger numbers in the developing brain than the other opioid receptors do, suggesting an important role in development [70-71]. For a review of the tissue distribution of this receptor see [67]. Spinal cord: dorsal and ventral horn. DRG: Found on large, medium, and small dorsal root ganglia neurons. |
||||||||
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
| Physiological Functions Comments | ||||||||
| Nociception: NOP receptor agonists attenuate opiate-mediated and stress-induced analgesia when administered i.c.v. but have antinociceptive activity when administered intrathecally. Small molecule agonists and antagonists generally have little effect on pain threshold when administered alone systemically in rodents. In chronic pain states systemic administration of small molecule NOP agonists is effective for inhibition of mechanical allodynia. In non-human primates, selective NOP agonists have antinociceptive activity. NOP receptor agonists and antagonists have many behavioural functions not discussed here, for a good review on the topic see [50]. For a review on the functional architecture of the NOP receptor, including information on splice variants, see reference [62]. |
||||||||
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Biologically Significant Variants 
|
||||||||||
|
||||||||||
|
1. Adapa ID, Toll L. (1997) Relationship between binding affinity and functional activity of nociceptin/orphanin FQ. Neuropeptides, 31 (5): 403-8. [PMID:9413015]
2. Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M, Bannister TD, Almli L, Stevens JS, Bradley B et al.. (2013) Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Sci Transl Med, 5 (188): 188ra73. [PMID:23740899]
3. Arcuri L, Novello S, Frassineti M, Mercatelli D, Pisanò CA, Morella I, Fasano S, Journigan BV, Meyer ME, Polgar WE et al.. (2018) Anti-Parkinsonian and anti-dyskinetic profiles of two novel potent and selective nociceptin/orphanin FQ receptor agonists. Br J Pharmacol, 175 (5): 782-796. [PMID:29232769]
4. Becker JA, Wallace A, Garzon A, Ingallinella P, Bianchi E, Cortese R, Simonin F, Kieffer BL, Pessi A. (1999) Ligands for kappa-opioid and ORL1 receptors identified from a conformationally constrained peptide combinatorial library. J Biol Chem, 274 (39): 27513-22. [PMID:10488086]
5. Berthele A, Platzer S, Dworzak D, Schadrack J, Mahal B, Büttner A, Assmus HP, Wurster K, Zieglgänsberger W, Conrad B et al.. (2003) [3H]-nociceptin ligand-binding and nociceptin opioid receptor mrna expression in the human brain. Neuroscience, 121 (3): 629-40. [PMID:14568023]
6. Berzetei-Gurske IP, Schwartz RW, Toll L. (1996) Determination of activity for nociceptin in the mouse vas deferens. Eur J Pharmacol, 302 (1-3): R1-2. [PMID:8791013]
7. Bigoni R, Giuliani S, Calo' G, Rizzi A, Guerrini R, Salvadori S, Regoli D, Maggi CA. (1999) Characterization of nociceptin receptors in the periphery: in vitro and in vivo studies. Naunyn Schmiedebergs Arch Pharmacol, 359 (3): 160-7. [PMID:10208302]
8. Bigoni R, Rizzi D, Rizzi A, Camarda V, Guerrini R, Lambert DG, Hashiba E, Berger H, Salvadori S, Regoli D et al.. (2002) Pharmacological characterisation of [(pX)Phe4]nociceptin(1-13)amide analogues. 1. In vitro studies. Naunyn Schmiedebergs Arch Pharmacol, 365 (6): 442-9. [PMID:12070757]
9. Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. (1994) Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett, 347 (2-3): 284-8. [PMID:8034019]
10. Calo G, Guerrini R, Rizzi A, Salvadori S, Burmeister M, Kapusta DR, Lambert DG, Regoli D. (2005) UFP-101, a peptide antagonist selective for the nociceptin/orphanin FQ receptor. CNS Drug Rev, 11: 97-112. [PMID:16007234]
11. Calo G, Rizzi A, Rizzi D, Bigoni R, Guerrini R, Marzola G, Marti M, McDonald J, Morari M, Lambert DG et al.. (2002) [Nphe1,Arg14,Lys15]nociceptin-NH2, a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. Br J Pharmacol, 136 (2): 303-11. [PMID:12010780]
12. Calo' G, Bigoni R, Rizzi A, Guerrini R, Salvadori S, Regoli D. (2000) Nociceptin/orphanin FQ receptor ligands. Peptides, 21 (7): 935-47. [PMID:10998527]
13. Calò G, Rizzi A, Bogoni G, Neugebauer V, Salvadori S, Guerrini R, Bianchi C, Regoli D. (1996) The mouse vas deferens: a pharmacological preparation sensitive to nociceptin. Eur J Pharmacol, 311 (1): R3-5. [PMID:8884244]
14. Camarda V, Fischetti C, Anzellotti N, Molinari P, Ambrosio C, Kostenis E, Regoli D, Trapella C, Guerrini R, Severo S et al.. (2009) Pharmacological profile of NOP receptors coupled with calcium signaling via the chimeric protein G alpha qi5. Naunyn Schmiedebergs Arch Pharmacol, 379 (6): 599-607. [PMID:19183962]
15. Carrà G, Rizzi A, Guerrini R, Barnes TA, McDonald J, Hebbes CP, Mela F, Kenigs VA, Marzola G, Rizzi D et al.. (2005) [(pF)Phe4,Arg14,Lys15]N/OFQ-NH2 (UFP-102), a highly potent and selective agonist of the nociceptin/orphanin FQ receptor. J Pharmacol Exp Ther, 312 (3): 1114-23. [PMID:15509719]
16. Chang SD, Mascarella SW, Spangler SM, Gurevich VV, Navarro HA, Carroll FI, Bruchas MR. (2015) Quantitative Signaling and Structure-Activity Analyses Demonstrate Functional Selectivity at the Nociceptin/Orphanin FQ Opioid Receptor. Mol Pharmacol, 88 (3): 502-11. [PMID:26134494]
17. Clarke S, Chen Z, Hsu MS, Hill RG, Pintar JE, Kitchen I. (2003) Nociceptin/orphanin FQ knockout mice display up-regulation of the opioid receptor-like 1 receptor and alterations in opioid receptor expression in the brain. Neuroscience, 117 (1): 157-68. [PMID:12605902]
18. Clarke S, Chen Z, Hsu MS, Pintar J, Hill R, Kitchen I. (2001) Quantitative autoradiographic mapping of the ORL1, mu-, delta- and kappa-receptors in the brains of knockout mice lacking the ORL1 receptor gene. Brain Res, 906: 13-24. [PMID:11430857]
19. Clarke S, Czyzyk T, Ansonoff M, Nitsche JF, Hsu MS, Nilsson L, Larsson K, Borsodi A, Toth G, Hill R, Kitchen I, Pintar JE. (2002) Autoradiography of opioid and ORL1 ligands in opioid receptor triple knockout mice. Eur J Neurosci, 16: 1705-1712. [PMID:12431223]
20. Connor M, Yeo A, Henderson G. (1996) The effect of nociceptin on Ca2+ channel current and intracellular Ca2+ in the SH-SY5Y human neuroblastoma cell line. Br J Pharmacol, 118 (2): 205-7. [PMID:8735615]
21. Depner UB, Reinscheid RK, Takeshima H, Brune K, Zeilhofer HU. (2003) Normal sensitivity to acute pain, but increased inflammatory hyperalgesia in mice lacking the nociceptin precursor polypeptide or the nociceptin receptor. Eur J Neurosci, 17 (11): 2381-7. [PMID:12814369]
22. Di Giannuario A, Pieretti S, Catalani A, Loizzo A. (1999) Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci Lett, 272 (3): 183-6. [PMID:10505611]
23. Ding H, Kiguchi N, Yasuda D, Daga PR, Polgar WE, Lu JJ, Czoty PW, Kishioka S, Zaveri NT, Ko MC. (2018) A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci Transl Med, 10 (456). [PMID:30158150]
24. Dooley CT, Spaeth CG, Berzetei-Gurske IP, Craymer K, Adapa ID, Brandt SR, Houghten RA, Toll L. (1997) Binding and in vitro activities of peptides with high affinity for the nociceptin/orphanin FQ receptor, ORL1. J Pharmacol Exp Ther, 283 (2): 735-41. [PMID:9353393]
25. Fernandez F, Misilmeri MA, Felger JC, Devine DP. (2004) Nociceptin/orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacology, 29 (1): 59-71. [PMID:14532912]
26. Ferrari F, Rizzo S, Ruzza C, Calo G. (2020) Detailed In Vitro Pharmacological Characterization of the Clinically Viable Nociceptin/Orphanin FQ Peptide Receptor Antagonist BTRX-246040. J Pharmacol Exp Ther, 373 (1): 34-43. [PMID:31937563]
27. Fischetti C, Camarda V, Rizzi A, Pelà M, Trapella C, Guerrini R, McDonald J, Lambert DG, Salvadori S, Regoli D et al.. (2009) Pharmacological characterization of the nociceptin/orphanin FQ receptor non peptide antagonist Compound 24. Eur J Pharmacol, 614 (1-3): 50-7. [PMID:19445927]
28. Florin S, Meunier J, Costentin J. (2000) Autoradiographic localization of [3H]nociceptin binding sites in the rat brain. Brain Res, 880 (1-2): 11-6. [PMID:11032985]
29. Gavioli EC, Marzola G, Guerrini R, Bertorelli R, Zucchini S, De Lima TC, Rae GA, Salvadori S, Regoli D, Calo G. (2003) Blockade of nociceptin/orphanin FQ-NOP receptor signalling produces antidepressant-like effects: pharmacological and genetic evidences from the mouse forced swimming test. Eur J Neurosci, 17 (9): 1987-90. [PMID:12752799]
30. Goto Y, Arai-Otsuki S, Tachibana Y, Ichikawa D, Ozaki S, Takahashi H, Iwasawa Y, Okamoto O, Okuda S, Ohta H et al.. (2006) Identification of a novel spiropiperidine opioid receptor-like 1 antagonist class by a focused library approach featuring 3D-pharmacophore similarity. J Med Chem, 49 (3): 847-9. [PMID:16451050]
31. Grisel JE, Mogil JS, Belknap JK, Grandy DK. (1996) Orphanin FQ acts as a supraspinal, but not a spinal, anti-opioid peptide. Neuroreport, 7 (13): 2125-9. [PMID:8930972]
32. Guerrini R, Calo G, Rizzi A, Bianchi C, Lazarus LH, Salvadori S, Temussi PA, Regoli D. (1997) Address and message sequences for the nociceptin receptor: a structure-activity study of nociceptin-(1-13)-peptide amide. J Med Chem, 40 (12): 1789-93. [PMID:9191955]
33. Guerrini R, Calo G, Rizzi A, Bigoni R, Bianchi C, Salvadori S, Regoli D. (1998) A new selective antagonist of the nociceptin receptor. Br J Pharmacol, 123 (2): 163-5. [PMID:9489602]
34. Guerrini R, Caló G, Lambert DG, Carrá G, Arduin M, Barnes TA, McDonald J, Rizzi D, Trapella C, Marzola E et al.. (2005) N- and C-terminal modifications of nociceptin/orphanin FQ generate highly potent NOP receptor ligands. J Med Chem, 48 (5): 1421-7. [PMID:15743186]
35. Hawes BE, Graziano MP, Lambert DG. (2000) Cellular actions of nociceptin: transduction mechanisms. Peptides, 21: 961-967. [PMID:10998529]
36. Hayashi S, Hirao A, Imai A, Nakamura H, Murata Y, Ohashi K, Nakata E. (2009) Novel non-peptide nociceptin/orphanin FQ receptor agonist, 1-[1-(1-Methylcyclooctyl)-4-piperidinyl]-2-[(3R)-3-piperidinyl]-1H-benzimidazole: design, synthesis, and structure-activity relationship of oral receptor occupancy in the brain for orally potent antianxiety drug. J Med Chem, 52 (3): 610-25. [PMID:19125610]
37. Ho M, Corbett AD, McKnight AT. (2000) Characterization of the ORL(1) receptor on adrenergic nerves in the rat anococcygeus muscle. Br J Pharmacol, 131 (2): 349-55. [PMID:10991930]
38. Inoue M, Kawashima T, Takeshima H, Calo G, Inoue A, Nakata Y, Ueda H. (2003) In vivo pain-inhibitory role of nociceptin/orphanin FQ in spinal cord. J Pharmacol Exp Ther, 305 (2): 495-501. [PMID:12606680]
39. Inoue M, Kobayashi M, Kozaki S, Zimmer A, Ueda H. (1998) Nociceptin/orphanin FQ-induced nociceptive responses through substance P release from peripheral nerve endings in mice. Proc Natl Acad Sci USA, 95 (18): 10949-53. [PMID:9724810]
40. Inoue M, Shimohira I, Yoshida A, Zimmer A, Takeshima H, Sakurada T, Ueda H. (1999) Dose-related opposite modulation by nociceptin/orphanin FQ of substance P nociception in the nociceptors and spinal cord. J Pharmacol Exp Ther, 291 (1): 308-13. [PMID:10490918]
41. Itoh K, Konya H, Takai E, Masuda H, Nagai K. (1999) Modification of acetylcholine release by nociceptin in conscious rat striatum. Brain Res, 845 (2): 242-5. [PMID:10536205]
42. Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma Jr FJ, Nothacker HP, Civelli O. (1997) Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci USA, 94 (26): 14854-8. [PMID:9405703]
43. Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, Lundstrom K, Cesura AM, Poli SM, Roever S et al.. (2000) A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci USA, 97 (9): 4938-43. [PMID:10758169]
44. Kapusta DR, Burmeister MA, Calo' G, Guerrini R, Gottlieb HB, Kenigs VA. (2005) Functional selectivity of nociceptin/orphanin FQ peptide receptor partial agonists on cardiovascular and renal function. J Pharmacol Exp Ther, 314 (2): 643-51. [PMID:15855356]
45. Kapusta DR, Kenigs VA. (1999) Cardiovascular and renal responses produced by central orphanin FQ/nociceptin occur independent of renal nerves. Am J Physiol, 277 (4): R987-95. [PMID:10516236]
46. Kawamoto H, Ozaki S, Itoh Y, Miyaji M, Arai S, Nakashima H, Kato T, Ohta H, Iwasawa Y. (1999) Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3- hydroxymethyl-4-piperidyl]-3-ethyl-1, 3-dihydro-2H-benzimidazol-2-one (J-113397). J Med Chem, 42 (25): 5061-3. [PMID:10602690]
47. Khroyan TV, Polgar WE, Cami-Kobeci G, Husbands SM, Zaveri NT, Toll L. (2011) The first universal opioid ligand, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028): characterization of the in vitro profile and in vivo behavioral effects in mouse models of acute pain and cocaine-induced reward. J Pharmacol Exp Ther, 336 (3): 952-61. [PMID:21177476]
48. Kiguchi N, Ding H, Cami-Kobeci G, Sukhtankar DD, Czoty PW, DeLoid HB, Hsu FC, Toll L, Husbands SM, Ko MC. (2019) BU10038 as a safe opioid analgesic with fewer side-effects after systemic and intrathecal administration in primates. Br J Anaesth, 122 (6): e146-e156. [PMID:30916003]
49. Konya H, Masuda H, Itoh K, Nagai K, Kakishita E, Matsuoka A. (1998) Modification of dopamine release by nociceptin in conscious rat striatum. Brain Res, 788 (1-2): 341-4. [PMID:9555088]
50. Lambert DG. (2008) The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov, 7 (8): 694-710. [PMID:18670432]
51. Linz K, Christoph T, Tzschentke TM, Koch T, Schiene K, Gautrois M, Schröder W, Kögel BY, Beier H, Englberger W et al.. (2014) Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther, 349 (3): 535-48. [PMID:24713140]
52. Malfacini D, Ambrosio C, Gro' MC, Sbraccia M, Trapella C, Guerrini R, Bonora M, Pinton P, Costa T, Calo' G. (2015) Pharmacological Profile of Nociceptin/Orphanin FQ Receptors Interacting with G-Proteins and β-Arrestins 2. PLoS ONE, 10 (8): e0132865. [PMID:26248189]
53. Manabe T, Noda Y, Mamiya T, Katagiri H, Houtani T, Nishi M, Noda T, Takahashi T, Sugimoto T, Nabeshima T et al.. (1998) Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature, 394 (6693): 577-81. [PMID:9707118]
54. Mandyam CD, Altememi GF, Standifer KM. (2000) beta-Funaltrexamine inactivates ORL1 receptors in BE(2)-C human neuroblastoma cells. Eur J Pharmacol, 402 (1-2): R1-37. [PMID:10940375]
55. Marti M, Mela F, Budri M, Volta M, Malfacini D, Molinari S, Zaveri NT, Ronzoni S, Petrillo P, Calò G et al.. (2013) Acute and chronic antiparkinsonian effects of the novel nociceptin/orphanin FQ receptor antagonist NiK-21273 in comparison with SB-612111. Br J Pharmacol, 168 (4): 863-79. [PMID:22994368]
56. Marti M, Mela F, Fantin M, Zucchini S, Brown JM, Witta J, Di Benedetto M, Buzas B, Reinscheid RK, Salvadori S et al.. (2005) Blockade of nociceptin/orphanin FQ transmission attenuates symptoms and neurodegeneration associated with Parkinson's disease. J Neurosci, 25 (42): 9591-601. [PMID:16237164]
57. Marti M, Mela F, Veronesi C, Guerrini R, Salvadori S, Federici M, Mercuri NB, Rizzi A, Franchi G, Beani L et al.. (2004) Blockade of nociceptin/orphanin FQ receptor signaling in rat substantia nigra pars reticulata stimulates nigrostriatal dopaminergic transmission and motor behavior. J Neurosci, 24 (30): 6659-66. [PMID:15282268]
58. Marti M, Stocchi S, Paganini F, Mela F, De Risi C, Calo' G, Guerrini R, Barnes TA, Lambert DG, Beani L et al.. (2003) Pharmacological profiles of presynaptic nociceptin/orphanin FQ receptors modulating 5-hydroxytryptamine and noradrenaline release in the rat neocortex. Br J Pharmacol, 138 (1): 91-8. [PMID:12522077]
59. McDonald J, Barnes TA, Okawa H, Williams J, Calo' G, Rowbotham DJ, Lambert DG. (2003) Partial agonist behaviour depends upon the level of nociceptin/orphanin FQ receptor expression: studies using the ecdysone-inducible mammalian expression system. Br J Pharmacol, 140 (1): 61-70. [PMID:12967935]
60. McLeod RL, Tulshian DB, Bolser DC, Varty GB, Baptista M, Fernandez X, Parra LE, Zimmer JC, Erickson CH, Ho GD et al.. (2010) Pharmacological profile of the NOP agonist and cough suppressing agent SCH 486757 (8-[Bis(2-Chlorophenyl)Methyl]-3-(2-Pyrimidinyl)-8-Azabicyclo[3.2.1]Octan-3-Ol) in preclinical models. Eur J Pharmacol, 630 (1-3): 112-20. [PMID:20006596]
61. Menzies JR, Glen T, Davies MR, Paterson SJ, Corbett AD. (1999) In vitro agonist effects of nociceptin and [Phe(1)psi(CH(2)-NH)Gly(2)]nociceptin(1-13)NH(2) in the mouse and rat colon and the mouse vas deferens. Eur J Pharmacol, 385 (2-3): 217-23. [PMID:10607879]
62. Meunier J, Mouledous L, Topham CM. (2000) The nociceptin (ORL1) receptor: molecular cloning and functional architecture. Peptides, 21 (7): 893-900. [PMID:10998522]
63. Meunier J-C, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B et al.. (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL-1 receptor. Nature, 377: 532-535. [PMID:7566152]
64. Mogil JS, Grisel JE, Zhangs G, Belknap JK, Grandy DK. (1996) Functional antagonism of mu-, delta- and kappa-opioid antinociception by orphanin FQ. Neurosci Lett, 214 (2-3): 131-4. [PMID:8878101]
65. Mogil JS, Pasternak GW. (2001) The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev, 53: 381-415. [PMID:11546835]
66. Mollereau C, Moisand C, Butour JL, Parmentier M, Meunier JC. (1996) Replacement of Gln280 by His in TM6 of the human ORL1 receptor increases affinity but reduces intrinsic activity of opioids. FEBS Lett, 395 (1): 17-21. [PMID:8849681]
67. Mollereau C, Mouledous L. (2000) Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides, 21 (7): 907-17. [PMID:10998524]
68. Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. (1994) ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett, 341 (1): 33-8. [PMID:8137918]
69. Murphy NP, Ly HT, Maidment NT. (1996) Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience, 75 (1): 1-4. [PMID:8923516]
70. Neal CR, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ. (1999) Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with125I-[14Tyr]-orphanin FQ binding. J Comp Neurol, 412: 563-605. [PMID:10464356]
71. Neal Jr CR, Akil H, Watson Jr SJ. (2001) Expression of orphanin FQ and the opioid receptor-like (ORL1) receptor in the developing human and rat brain. J Chem Neuroanat, 22 (4): 219-49. [PMID:11719021]
72. Nicol B, Lambert DG, Rowbotham DJ, Smart D, McKnight AT. (1996) Nociceptin induced inhibition of K+ evoked glutamate release from rat cerebrocortical slices. Br J Pharmacol, 119 (6): 1081-3. [PMID:8937708]
73. Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, Kuno J, Takeshima H, Nukada T, Nabeshima T et al.. (1997) Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J, 16 (8): 1858-64. [PMID:9155012]
74. Okada K, Sujaku T, Chuman Y, Nakashima R, Nose T, Costa T, Yamada Y, Yokoyama M, Nagahisa A, Shimohigashi Y. (2000) Highly potent nociceptin analog containing the Arg-Lys triple repeat. Biochem Biophys Res Commun, 278: 493-498. [PMID:11097863]
75. Okawa H, Nicol B, Bigoni R, Hirst RA, Calo G, Guerrini R, Rowbotham DJ, Smart D, McKnight AT, Lambert DG. (1999) Comparison of the effects of [Phe1psi(CH2-NH)Gly2]nociceptin(1-13)NH2 in rat brain, rat vas deferens and CHO cells expressing recombinant human nociceptin receptors. Br J Pharmacol, 127 (1): 123-30. [PMID:10369464]
76. Ozaki S, Kawamoto H, Itoh Y, Miyaji M, Azuma T, Ichikawa D, Nambu H, Iguchi T, Iwasawa Y, Ohta H. (2000) In vitro and in vivo pharmacological characterization of J-113397, a potent and selective non-peptidyl ORL1 receptor antagonist. Eur J Pharmacol, 402 (1-2): 45-53. [PMID:10940356]
77. Pan YX, Cheng J, Xu J, Rossi G, Jacobson E, Ryan-Moro J, Brooks AI, Dean GE, Standifer KM, Pasternak GW. (1995) Cloning and functional characterization through antisense mapping of a kappa 3-related opioid receptor. Mol Pharmacol, 47 (6): 1180-8. [PMID:7603458]
78. Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma Jr FJ, Civelli O. (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science, 270 (5237): 792-4. [PMID:7481766]
79. Rizzi A, Malfacini D, Cerlesi MC, Ruzza C, Marzola E, Bird MF, Rowbotham DJ, Salvadori S, Guerrini R, Lambert DG et al.. (2014) In vitro and in vivo pharmacological characterization of nociceptin/orphanin FQ tetrabranched derivatives. Br J Pharmacol, 171 (17): 4138-53. [PMID:24903280]
80. Rizzi A, Spagnolo B, Wainford RD, Fischetti C, Guerrini R, Marzola G, Baldisserotto A, Salvadori S, Regoli D, Kapusta DR et al.. (2007) In vitro and in vivo studies on UFP-112, a novel potent and long lasting agonist selective for the nociceptin/orphanin FQ receptor. Peptides, 28 (6): 1240-51. [PMID:17532097]
81. Schlicker E, Werthwein S, Kathmann M, Bauer U. (1998) Nociceptin inhibits noradrenaline release in the mouse brain cortex via presynaptic ORL1 receptors. Naunyn Schmiedebergs Arch Pharmacol, 358 (4): 418-22. [PMID:9826063]
82. Shinkai H, Ito T, Iida T, Kitao Y, Yamada H, Uchida I. (2000) 4-Aminoquinolines: novel nociceptin antagonists with analgesic activity. J Med Chem, 43 (24): 4667-77. [PMID:11101358]
83. Sim LJ, Xiao R, Childers SR. (1996) Identification of opioid receptor-like (ORL1) peptide-stimulated [35S]GTP gamma S binding in rat brain. Neuroreport, 7 (3): 729-33. [PMID:8733732]
84. Slowe SJ, Clarke S, Lena I, Goody RJ, Lattanzi R, Negri L, Simonin F, Matthes HW, Filliol D, Kieffer BL, Kitchen I. (2001) Autoradiographic mapping of the opioid receptor-like 1 (ORL1) receptor in the brains of mu-, delta- or kappa-opioid receptor knockout mice. Neuroscience, 106: 469-480. [PMID:11591451]
85. Spagnolo B, Carrà G, Fantin M, Fischetti C, Hebbes C, McDonald J, Barnes TA, Rizzi A, Trapella C, Fanton G et al.. (2007) Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol]: in vitro studies. J Pharmacol Exp Ther, 321 (3): 961-7. [PMID:17329552]
86. Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, Huang XP, Trapella C, Guerrini R, Calo G et al.. (2012) Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature, 485 (7398): 395-9. [PMID:22596163]
87. Toledo MA, Pedregal C, Lafuente C, Diaz N, Martinez-Grau MA, Jiménez A, Benito A, Torrado A, Mateos C, Joshi EM et al.. (2014) Discovery of a novel series of orally active nociceptin/orphanin FQ (NOP) receptor antagonists based on a dihydrospiro(piperidine-4,7'-thieno[2,3-c]pyran) scaffold. J Med Chem, 57 (8): 3418-29. [PMID:24678969]
88. Toll L, Khroyan TV, Polgar WE, Jiang F, Olsen C, Zaveri NT. (2009) Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/mu-opioid receptor ligands: implications for therapeutic applications. J Pharmacol Exp Ther, 331 (3): 954-64. [PMID:19773529]
89. Trapella C, Guerrini R, Piccagli L, Calo' G, Carra' G, Spagnolo B, Rubini S, Fanton G, Hebbes C, McDonald J et al.. (2006) Identification of an achiral analogue of J-113397 as potent nociceptin/orphanin FQ receptor antagonist. Bioorg Med Chem, 14 (3): 692-704. [PMID:16202610]
90. Ueda H, Inoue M, Takeshima H, Iwasawa Y. (2000) Enhanced spinal nociceptin receptor expression develops morphine tolerance and dependence. J Neurosci, 20 (20): 7640-7. [PMID:11027224]
91. Varty GB, Lu SX, Morgan CA, Cohen-Williams ME, Hodgson RA, Smith-Torhan A, Zhang H, Fawzi AB, Graziano MP, Ho GD et al.. (2008) The anxiolytic-like effects of the novel, orally active nociceptin opioid receptor agonist 8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol (SCH 221510). J Pharmacol Exp Ther, 326 (2): 672-82. [PMID:18492950]
92. Wang HL, Hsu CY, Huang PC, Kuo YL, Li AH, Yeh TH, Tso AS, Chen YL. (2005) Heterodimerization of opioid receptor-like 1 and mu-opioid receptors impairs the potency of micro receptor agonist. J Neurochem, 92 (6): 1285-94. [PMID:15748148]
93. Wang JB, Johnson PS, Imai Y, Persico AM, Ozenberger BA, Eppler CM, Uhl GR. (1994) cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett, 348 (1): 75-9. [PMID:8026588]
94. Whiteside GT, Kyle DJ, Kapil RP, Cipriano A, He E, Zhou M, Shet MS, Hummel M, Knappenberger T, Fukumura K et al.. (2024) The nociceptin/orphanin FQ receptor partial agonist sunobinop promotes non-REM sleep in rodents and patients with insomnia. J Clin Invest, 134 (1). [PMID:37883189]
95. Wichmann J, Adam G, Röver S, Cesura AM, Dautzenberg FM, Jenck F. (1999) 8-acenaphthen-1-yl-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one derivatives as orphanin FQ receptor agonists. Bioorg Med Chem Lett, 9 (16): 2343-8. [PMID:10476866]
96. Wichmann J, Adam G, Röver S, Hennig M, Scalone M, Cesura AM, Dautzenberg FM, Jenck F. (2000) Synthesis of (1S,3aS)-8-(2,3,3a,4,5, 6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4. 5]decan-4-one, a potent and selective orphanin FQ (OFQ) receptor agonist with anxiolytic-like properties. Eur J Med Chem, 35 (9): 839-51. [PMID:11006485]
97. Xu XJ, Hao JX, Wiesenfeld-Hallin Z. (1996) Nociceptin or antinociceptin: potent spinal antinociceptive effect of orphanin FQ/nociceptin in the rat. Neuroreport, 7 (13): 2092-4. [PMID:8930965]
98. Zaratin PF, Petrone G, Sbacchi M, Garnier M, Fossati C, Petrillo P, Ronzoni S, Giardina GA, Scheideler MA. (2004) Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111). J Pharmacol Exp Ther, 308 (2): 454-61. [PMID:14593080]
99. Zaveri N. (2003) Peptide and nonpeptide ligands for the nociceptin/orphanin FQ receptor ORL1: research tools and potential therapeutic agents. Life Sci, 73: 663-678. [PMID:12801588]
100. Zaveri NT, Journigan VB, Polgar WE. (2015) Discovery of the first small-molecule opioid pan antagonist with nanomolar affinity at mu, delta, kappa, and nociceptin opioid receptors. ACS Chem Neurosci, 6 (4): 646-57. [PMID:25635572]