
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
 target has curated data in GtoImmuPdb
target has curated data in GtoImmuPdb
Target id: 2829
Nomenclature: indoleamine 2,3-dioxygenase 1
Abbreviated Name: IDO1
Family: 1.13.11.- Dioxygenases
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 403 | 8p11.21 | IDO1 | indoleamine 2,3-dioxygenase 1 | 2,20 |
| Mouse | - | 407 | 8 A2 | Ido1 | indoleamine 2,3-dioxygenase 1 | |
| Rat | - | 407 | 16q12.5 | Ido1 | indoleamine 2,3-dioxygenase 1 | |
| Gene and Protein Information Comments | ||||||
| Two isoforms of mouse Ido1 have been identified. We list the longer of the two, isoform 1 in the table above. The shorter isoform has only 316 amino acids. | ||||||
Previous and Unofficial Names  |
| INDO | indoleamine-pyrrole 2,3 dioxygenase | IDO-1 | IDO | indoleamine 2 |
Database Links  |
|
| Alphafold | P14902 (Hs), P28776 (Mm), Q9ERD9 (Rn) |
| BRENDA | 1.13.11.52 |
| ChEMBL Target | CHEMBL4685 (Hs), CHEMBL1075294 (Mm), CHEMBL4523451 (Rn) |
| Ensembl Gene | ENSG00000131203 (Hs), ENSMUSG00000031551 (Mm), ENSRNOG00000031189 (Rn) |
| Entrez Gene | 3620 (Hs), 15930 (Mm), 66029 (Rn) |
| Human Protein Atlas | ENSG00000131203 (Hs) |
| KEGG Enzyme | 1.13.11.52 |
| KEGG Gene | hsa:3620 (Hs), mmu:15930 (Mm), rno:66029 (Rn) |
| OMIM | 147435 (Hs) |
| Pharos | P14902 (Hs) |
| RefSeq Nucleotide | NM_002164 (Hs), NM_008324 (Mm), NM_001293690 (Mm), NM_023973 (Rn) |
| RefSeq Protein | NP_002155 (Hs), NP_001280619 (Mm), NP_032350 (Mm), NP_076463 (Rn) |
| SynPHARM | 83385 (in complex with NLG919) |
| UniProtKB | P14902 (Hs), P28776 (Mm), Q9ERD9 (Rn) |
| Wikipedia | IDO1 (Hs) |
Selected 3D Structures  |
|||||||||||||
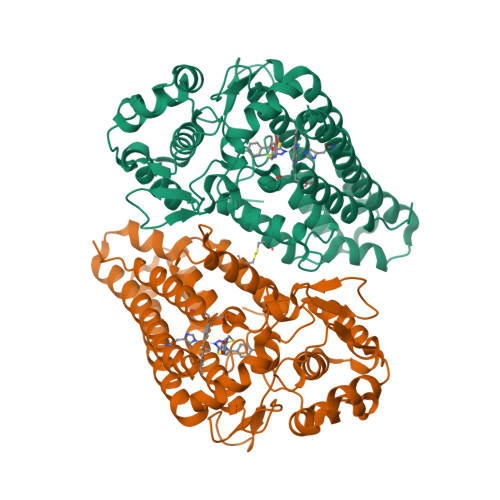
|
|
||||||||||||
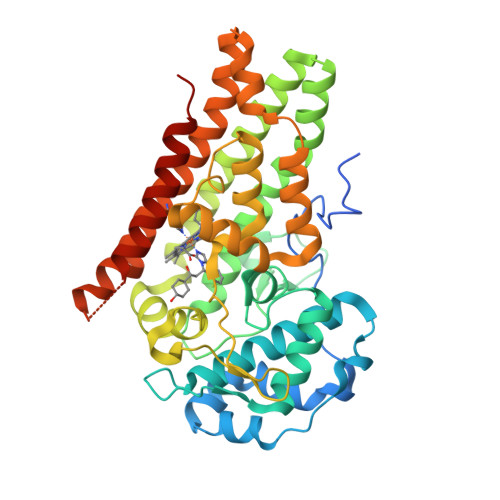
|
|
||||||||||||
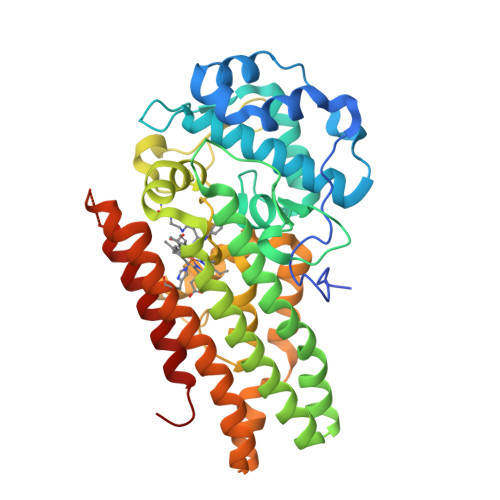
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| The indoleamine 2,3 dioxygenase (IDO) branch of the kynurenine (KYN) pathway of tryptophan metabolism has been intensely studied in relation to immune tolerance and allergy. IDO is generally considered to be a tolerogenic, immunosuppressive enzyme, that is induced by IFN-γ. It provides a negative feedback pathway that limits uncontrolled immune responses. Its immunosuppressive actions arise from its promotion of tryptophan depletion, and elevation of KYN metabolite levels. The aryl hydrocarbon receptor serves as a receptor for KYN and should be considered when evaluating the IDO-KYN pathway in immune homeostasis and its potential to modulate innate and adaptive immune responses [9]. IDO1 expression is increased by IFN-γ and IL-6 [12] (the latter in the presence of a histone deacetylase inhibitor). It is proposed that upregulating IDO1 via HDAC inhibition may be a therapeutic approach for the acute lethal idiopathic pulmonary syndrome that is a complication following allogeneic hematopoietic stem cell transplantation [12]. In the tumour microenvironment, depletion of tryptophan (catalysed by tumour-expressed IDO1) promotes Treg cell differentiation, suppresses the immune response and decreases dendritic cell function, thus creating a tolerogenic environment in which the cancer cells can survive and proliferate. Since IDO1 inhibitors have been shown to reverse this escape mechanism, ID01 has emerged as a promising molecular target for the development of novel agents as cancer immunotherapeutics [7], as well as for the treatment of other diseases that are characterised by the reduction of local tryptophan levels. The applicability of ID01 inhibition has been shown in preclinical models of diseases including arthritis, ischemia-reperfusion injury, endotoxin shock, human immunodeficiency virus (HIV)/simian immunodeficiency virus (SIV) infection, airway inflammation, and cancer [6,25]. However, clinical therapeutic efficacy has proven elusive [10]. |
| Cell Type Associations | ||||||
|
| Immuno Process Associations | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
| General Comments |
|
IDO1 is a rate-limiting enzyme of tryptophan catabolism along the kynurenine pathway. IDO1 catabolises both the L- and D- enantiomers of tryptophan, but shows preference for L-tryptophan. Additional IDO1 substrates include indoleamines such as serotonin, melatonin, and tryptamine [22]. IDO1 emerged as an important new therapeutic target for the treatment of cancer, neurological disorders, and other diseases that are characterized by pathological tryptophan metabolism. Despite encouraging anti-tumour activity in preclinical models and good tolerability and encouraging efficacy in early stage clinical trials, especially in combination with immune checkpoint inhibitors [18], the potential of IDO1 inhibitors may not be translated to later stage clinical efficacy. Results from the Phase 3 ECHO-301 study (NCT02752074) found no evidence that epacadostat provides any increased benefit compared to pembrolizumab alone in patients with unresectable or metastatic melanoma [14]. This failure has led to a re-evaluation of the utility of IDO1 inhibition as an adjunct to checkpoint inhibition in immuno-oncology [23] (see also IDO Inhibitors Hit a Wall on Derek Lowe's In The Pipeline blog, April 9, 2018). |
1. Crosignani S, Cauwenberghs S, Driessens G, Deroose F. (2015) Pyrrolidine-2,5-dione derivatives, pharmaceutical compositions and methods for use as ido1 inhibitors. Patent number: WO2015173764. Assignee: Iteos Therapeutics. Priority date: 15/05/2014. Publication date: 19/11/2015.
2. Dai W, Gupta SL. (1990) Molecular cloning, sequencing and expression of human interferon-gamma-inducible indoleamine 2,3-dioxygenase cDNA. Biochem Biophys Res Commun, 168 (1): 1-8. [PMID:2109605]
3. Dolušić E, Larrieu P, Blanc S, Sapunaric F, Norberg B, Moineaux L, Colette D, Stroobant V, Pilotte L, Colau D et al.. (2011) Indol-2-yl ethanones as novel indoleamine 2,3-dioxygenase (IDO) inhibitors. Bioorg Med Chem, 19 (4): 1550-61. [PMID:21269836]
4. Fu R, Zhang YW, Li HM, Lv WC, Zhao L, Guo QL, Lu T, Weiss SJ, Li ZY, Wu ZQ. (2018) LW106, a novel indoleamine 2,3-dioxygenase 1 inhibitor, suppresses tumour progression by limiting stroma-immune crosstalk and cancer stem cell enrichment in tumour micro-environment. Br J Pharmacol, 175 (14): 3034-3049. [PMID:29722898]
5. Heitger A. (2011) Regulation of expression and function of IDO in human dendritic cells. Curr Med Chem, 18 (15): 2222-33. [PMID:21517757]
6. Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. (2013) Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med, 210 (7): 1389-402. [PMID:23752227]
7. Hornyák L, Dobos N, Koncz G, Karányi Z, Páll D, Szabó Z, Halmos G, Székvölgyi L. (2018) The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Front Immunol, 9: 151. [PMID:29445380]
8. Huang R, Jing X, Huang X, Pan Y, Fang Y, Liang G, Liao Z, Wang H, Chen Z, Zhang Y. (2020) Bifunctional Naphthoquinone Aromatic Amide-Oxime Derivatives Exert Combined Immunotherapeutic and Antitumor Effects through Simultaneous Targeting of Indoleamine-2,3-dioxygenase and Signal Transducer and Activator of Transcription 3. J Med Chem, 63 (4): 1544-1563. [PMID:31999451]
9. Koch S, Stroisch TJ, Vorac J, Herrmann N, Leib N, Schnautz S, Kirins H, Förster I, Weighardt H, Bieber T. (2017) AhR mediates an anti-inflammatory feedback mechanism in human Langerhans cells involving FcεRI and IDO. Allergy, 72 (11): 1686-1693. [PMID:28376268]
10. Komiya T, Huang CH. (2018) Updates in the Clinical Development of Epacadostat and Other Indoleamine 2,3-Dioxygenase 1 Inhibitors (IDO1) for Human Cancers. Front Oncol, 8: 423. [PMID:30338242]
11. Kumar S, Waldo JP, Jaipuri FA, Marcinowicz A, Van Allen C, Adams J, Kesharwani T, Zhang X, Metz R, Oh AJ et al.. (2019) Discovery of Clinical Candidate (1R,4r)-4-((R)-2-((S)-6-Fluoro-5H-imidazo[5,1-a]isoindol-5-yl)-1-hydroxyethyl)cyclohexan-1-ol (Navoximod), a Potent and Selective Inhibitor of Indoleamine 2,3-Dioxygenase 1. J Med Chem, 62 (14): 6705-6733. [PMID:31264862]
12. Lee SM, Park HY, Suh YS, Yoon EH, Kim J, Jang WH, Lee WS, Park SG, Choi IW, Choi I et al.. (2017) Inhibition of acute lethal pulmonary inflammation by the IDO-AhR pathway. Proc Natl Acad Sci USA, 114 (29): E5881-E5890. [PMID:28673995]
13. Li J, Li Y, Yang D, Hu N, Guo Z, Kuang C, Yang Q. (2016) Establishment of a human indoleamine 2, 3-dioxygenase 2 (hIDO2) bioassay system and discovery of tryptanthrin derivatives as potent hIDO2 inhibitors. Eur J Med Chem, 123: 171-179. [PMID:27475108]
14. Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, Arance A, Carlino MS, Grob JJ, Kim TM et al.. (2019) Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol, 20 (8): 1083-1097. [PMID:31221619]
15. Luo S, Xu K, Xiang S, Chen J, Chen C, Guo C, Tong Y, Tong L. (2018) High-resolution structures of inhibitor complexes of human indoleamine 2,3-dioxygenase 1 in a new crystal form. Acta Crystallogr F Struct Biol Commun, 74 (Pt 11): 717-724. [PMID:30387777]
16. Meininger D, Zalameda L, Liu Y, Stepan LP, Borges L, McCarter JD, Sutherland CL. (2011) Purification and kinetic characterization of human indoleamine 2,3-dioxygenases 1 and 2 (IDO1 and IDO2) and discovery of selective IDO1 inhibitors. Biochim Biophys Acta, 1814 (12): 1947-54. [PMID:21835273]
17. Mellor AL, Munn DH. (1999) Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation?. Immunol Today, 20 (10): 469-73. [PMID:10500295]
18. Mitchell TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Olszanski AJ, Luke JJ, Balmanoukian AS, Schmidt EV, Zhao Y et al.. (2018) Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J Clin Oncol, 36 (32): 3223-3230. [PMID:30265610]
19. Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. (2005) Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med, 11 (3): 312-9. [PMID:15711557]
20. Najfeld V, Menninger J, Muhleman D, Comings DE, Gupta SL. (1993) Localization of indoleamine 2,3-dioxygenase gene (INDO) to chromosome 8p12-->p11 by fluorescent in situ hybridization. Cytogenet Cell Genet, 64 (3-4): 231-2. [PMID:8404046]
21. Nelp MT, Kates PA, Hunt JT, Newitt JA, Balog A, Maley D, Zhu X, Abell L, Allentoff A, Borzilleri R et al.. (2018) Immune-modulating enzyme indoleamine 2,3-dioxygenase is effectively inhibited by targeting its apo-form. Proc Natl Acad Sci USA, 115 (13): 3249-3254. [PMID:29531094]
22. Shimizu T, Nomiyama S, Hirata F, Hayaishi O. (1978) Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem, 253 (13): 4700-6. [PMID:26687]
23. Sondak VK, Khushalani NI. (2019) Echoes of a failure: what lessons can we learn?. Lancet Oncol, 20 (8): 1037-1039. [PMID:31221617]
24. Tojo S, Kohno T, Tanaka T, Kamioka S, Ota Y, Ishii T, Kamimoto K, Asano S, Isobe Y. (2014) Crystal Structures and Structure-Activity Relationships of Imidazothiazole Derivatives as IDO1 Inhibitors. ACS Med Chem Lett, 5 (10): 1119-23. [PMID:25313323]
25. Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med, 9 (10): 1269-74. [PMID:14502282]
26. Yang S, Li X, Hu F, Li Y, Yang Y, Yan J, Kuang C, Yang Q. (2013) Discovery of tryptanthrin derivatives as potent inhibitors of indoleamine 2,3-dioxygenase with therapeutic activity in Lewis lung cancer (LLC) tumor-bearing mice. J Med Chem, 56 (21): 8321-31. [PMID:24099220]
27. Yue EW, Douty B, Wayland B, Bower M, Liu X, Leffet L, Wang Q, Bowman KJ, Hansbury MJ, Liu C et al.. (2009) Discovery of potent competitive inhibitors of indoleamine 2,3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model. J Med Chem, 52 (23): 7364-7. [PMID:19507862]
1.13.11.- Dioxygenases: indoleamine 2,3-dioxygenase 1. Last modified on 18/03/2020. Accessed on 01/07/2025. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2829.