
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Gene and Protein Information  |
|||||||
| Species | TM | P Loops | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 2 | 1 | 501 | 2q24.1 | KCNJ3 | potassium inwardly rectifying channel subfamily J member 3 | 44 |
| Mouse | 2 | 1 | 501 | 2 C1.1 | Kcnj3 | potassium inwardly-rectifying channel, subfamily J, member 3 | 21,47 |
| Rat | 2 | 1 | 501 | 3q21 | Kcnj3 | potassium inwardly-rectifying channel, subfamily J, member 3 | 5,26 |
Database Links  |
|
| Alphafold | P48549 (Hs), P63250 (Mm), P63251 (Rn) |
| CATH/Gene3D | 2.60.40.1400 |
| ChEMBL Target | CHEMBL1914277 (Hs), CHEMBL3832643 (Rn) |
| Ensembl Gene | ENSG00000162989 (Hs), ENSMUSG00000026824 (Mm), ENSRNOG00000005369 (Rn) |
| Entrez Gene | 3760 (Hs), 16519 (Mm), 50599 (Rn) |
| Human Protein Atlas | ENSG00000162989 (Hs) |
| KEGG Gene | hsa:3760 (Hs), mmu:16519 (Mm), rno:50599 (Rn) |
| OMIM | 601534 (Hs) |
| Pharos | P48549 (Hs) |
| RefSeq Nucleotide | NM_002239 (Hs), NM_008426 (Mm), NM_031610 (Rn) |
| RefSeq Protein | NP_002230 (Hs), NP_032452 (Mm), NP_113798 (Rn) |
| UniProtKB | P48549 (Hs), P63250 (Mm), P63251 (Rn) |
| Wikipedia | KCNJ3 (Hs) |
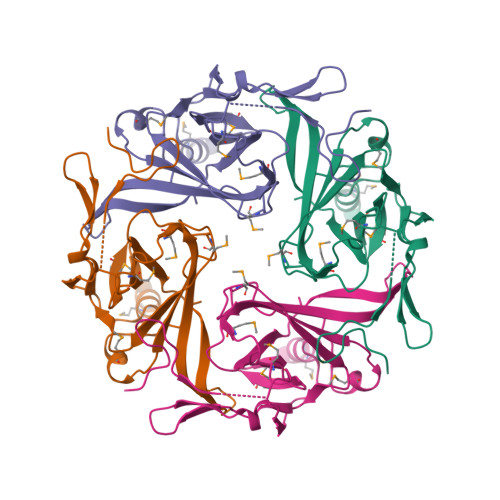
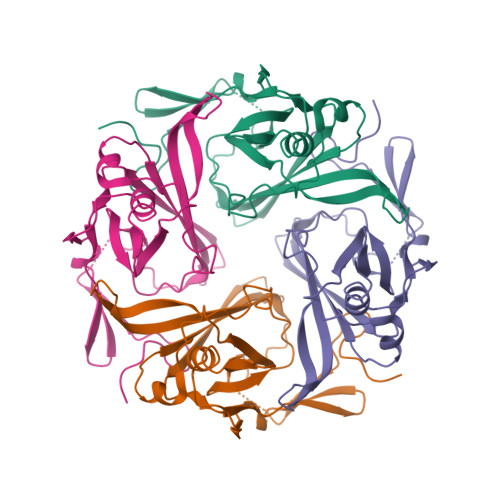
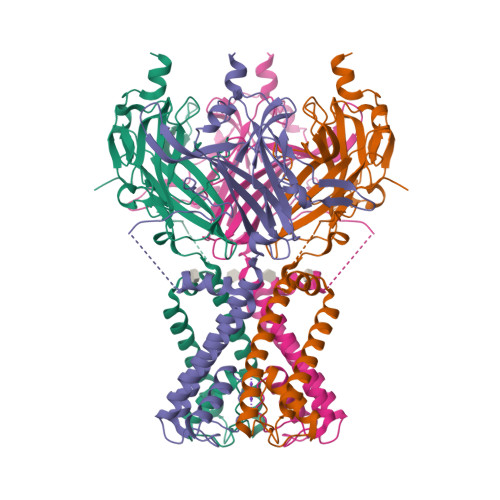
Selected 3D Structures  |
|||||||||||
|
|
||||||||||
|
|
||||||||||
|
|
||||||||||
Associated Proteins  |
||||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||
| Associated Protein Comments | ||||||||||||||||||||||||||||||||
| Kir3.1 is not functional alone. The functional expression of Kir3.1 in Xenopus oocytes requires coassembly with the endogenous Xenopus Kir3.5 subunit [11]. The major functional assembly in the heart is the Kir3.1/3.4 heteromultimer [25], while in the brain it is Kir3.1/3.2, Kir3.1/3.3 and Kir3.2/3.3 [1,3-4,7,23-24,28-29,33,46]. | ||||||||||||||||||||||||||||||||
Functional Characteristics  |
|
| G protein-activated inward-rectifier current | |
Ion Selectivity and Conductance  |
||||||
|
||||||
| Ion Selectivity and Conductance Comments | ||||||
| Please see [27,34] for review. Kir3.1 forms functional heteromers with Kir3.3 (39pS, [15]) and Kir3.4 (K+, 36.6pS [25]). |
Download all structure-activity data for this target as a CSV file 
| Activators | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific activator tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Activator Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kir3.1 is also activated by Gβγ [13-14,27,42,48] and modulated by RGS8 [43] and RGS2 [6]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gating Inhibitor Comments | ||
| Gα subunits inhibit the receptor by reducing the availability of Gβγ subunits for activation [45]. Gα subunits bind to Kir3.1 [40]. ER retention motif identified in K3.1 [35]. SNX27 regulates trafficking of Kir3.1 and Kir3.3 channels [32]. |
| Channel Blockers | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific channel blocker tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Channel Blocker Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| These studies were performed using Kir3.1 expressed in Xenopus oocytes. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
| Physiological Functions Comments | |
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||
|
|||||||||||||||||||
1. Aguado C, Colón J, Ciruela F, Schlaudraff F, Cabañero MJ, Perry C, Watanabe M, Liss B, Wickman K, Luján R. (2008) Cell type-specific subunit composition of G protein-gated potassium channels in the cerebellum. J Neurochem, 105 (2): 497-511. [PMID:18088366]
2. AstraZeneca. AZD2927. Accessed on 11/09/2014. Modified on 11/09/2014. astrazeneca.com, http://openinnovation.astrazeneca.com/what-we-offer/compound/azd2927/
3. Ciruela F, Fernández-Dueñas V, Sahlholm K, Fernández-Alacid L, Nicolau JC, Watanabe M, Luján R. (2010) Evidence for oligomerization between GABAB receptors and GIRK channels containing the GIRK1 and GIRK3 subunits. Eur J Neurosci, 32 (8): 1265-77. [PMID:20846323]
4. Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Lüscher C. (2004) Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci, 7 (2): 153-9. [PMID:14745451]
5. Dascal N, Schreibmayer W, Lim NF, Wang W, Chavkin C, DiMagno L, Labarca C, Kieffer BL, Gaveriaux-Ruff C, Trollinger D et al.. (1993) Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc Natl Acad Sci USA, 90 (21): 10235-9. [PMID:8234283]
6. Doupnik CA, Davidson N, Lester HA, Kofuji P. (1997) RGS proteins reconstitute the rapid gating kinetics of gbetagamma-activated inwardly rectifying K+ channels. Proc Natl Acad Sci USA, 94 (19): 10461-6. [PMID:9294233]
7. Fernández-Alacid L, Aguado C, Ciruela F, Martín R, Colón J, Cabañero MJ, Gassmann M, Watanabe M, Shigemoto R, Wickman K et al.. (2009) Subcellular compartment-specific molecular diversity of pre- and post-synaptic GABA-activated GIRK channels in Purkinje cells. J Neurochem, 110 (4): 1363-76. [PMID:19558451]
8. Fowler CE, Aryal P, Suen KF, Slesinger PA. (2007) Evidence for association of GABA(B) receptors with Kir3 channels and regulators of G protein signalling (RGS4) proteins. J Physiol (Lond.), 580 (Pt 1): 51-65. [PMID:17185339]
9. Fujita S, Inanobe A, Chachin M, Aizawa Y, Kurachi Y. (2000) A regulator of G protein signalling (RGS) protein confers agonist-dependent relaxation gating to a G protein-gated K+ channel. J Physiol (Lond.), 526 Pt 2: 341-7. [PMID:10896722]
10. Hearing M, Kotecki L, Marron Fernandez de Velasco E, Fajardo-Serrano A, Chung HJ, Luján R, Wickman K. (2013) Repeated cocaine weakens GABA(B)-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron, 80 (1): 159-70. [PMID:24094109]
11. Hedin KE, Lim NF, Clapham DE. (1996) Cloning of a Xenopus laevis inwardly rectifying K+ channel subunit that permits GIRK1 expression of IKACh currents in oocytes. Neuron, 16 (2): 423-9. [PMID:8789957]
12. Ho IH, Murrell-Lagnado RD. (1999) Molecular determinants for sodium-dependent activation of G protein-gated K+ channels. J Biol Chem, 274 (13): 8639-48. [PMID:10085101]
13. Huang CL, Feng S, Hilgemann DW. (1998) Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature, 391 (6669): 803-6. [PMID:9486652]
14. Huang CL, Slesinger PA, Casey PJ, Jan YN, Jan LY. (1995) Evidence that direct binding of G beta gamma to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron, 15 (5): 1133-43. [PMID:7576656]
15. Jelacic TM, Sims SM, Clapham DE. (1999) Functional expression and characterization of G-protein-gated inwardly rectifying K+ channels containing GIRK3. J Membr Biol, 169 (2): 123-9. [PMID:10341034]
16. Jin W, Klem AM, Lewis JH, Lu Z. (1999) Mechanisms of inward-rectifier K+ channel inhibition by tertiapin-Q. Biochemistry, 38 (43): 14294-301. [PMID:10572004]
17. Jin W, Lu Z. (1998) A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry, 37 (38): 13291-9. [PMID:9748337]
18. Karschin C, Dissmann E, Stühmer W, Karschin A. (1996) IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci, 16 (11): 3559-70. [PMID:8642402]
19. Karschin C, Karschin A. (1997) Ontogeny of gene expression of Kir channel subunits in the rat. Mol Cell Neurosci, 10 (3-4): 131-48. [PMID:9532576]
20. Kaufmann K, Romaine I, Days E, Pascual C, Malik A, Yang L, Zou B, Du Y, Sliwoski G, Morrison RD et al.. (2013) ML297 (VU0456810), the first potent and selective activator of the GIRK potassium channel, displays antiepileptic properties in mice. ACS Chem Neurosci, 4 (9): 1278-86. [PMID:23730969]
21. Kobayashi T, Ikeda K, Ichikawa T, Abe S, Togashi S, Kumanishi T. (1995) Molecular cloning of a mouse G-protein-activated K+ channel (mGIRK1) and distinct distributions of three GIRK (GIRK1, 2 and 3) mRNAs in mouse brain. Biochem Biophys Res Commun, 208 (3): 1166-73. [PMID:7702616]
22. Kobayashi T, Ikeda K, Kojima H, Niki H, Yano R, Yoshioka T, Kumanishi T. (1999) Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat Neurosci, 2 (12): 1091-7. [PMID:10570486]
23. Kofuji P, Davidson N, Lester HA. (1995) Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers. Proc Natl Acad Sci USA, 92 (14): 6542-6. [PMID:7604029]
24. Koyrakh L, Luján R, Colón J, Karschin C, Kurachi Y, Karschin A, Wickman K. (2005) Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J Neurosci, 25 (49): 11468-78. [PMID:16339040]
25. Krapivinsky G, Gordon EA, Wickman K, Velimirović B, Krapivinsky L, Clapham DE. (1995) The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature, 374 (6518): 135-41. [PMID:7877685]
26. Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan LY. (1993) Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature, 364 (6440): 802-6. [PMID:8355805]
27. Kurachi Y. (1995) G protein regulation of cardiac muscarinic potassium channel. Am J Physiol, 269 (4 Pt 1): C821-30. [PMID:7485449]
28. Leaney JL. (2003) Contribution of Kir3.1, Kir3.2A and Kir3.2C subunits to native G protein-gated inwardly rectifying potassium currents in cultured hippocampal neurons. Eur J Neurosci, 18 (8): 2110-8. [PMID:14622172]
29. Lesage F, Guillemare E, Fink M, Duprat F, Heurteaux C, Fosset M, Romey G, Barhanin J, Lazdunski M. (1995) Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem, 270 (48): 28660-7. [PMID:7499385]
30. Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. (1999) G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci, 2 (12): 1084-90. [PMID:10570485]
31. Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. (1987) The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature, 325 (6102): 321-6. [PMID:2433589]
32. Lunn ML, Nassirpour R, Arrabit C, Tan J, McLeod I, Arias CM, Sawchenko PE, Yates 3rd JR, Slesinger PA. (2007) A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat Neurosci, 10 (10): 1249-59. [PMID:17828261]
33. Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. (1997) G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron, 19 (3): 687-95. [PMID:9331358]
34. Lüscher C, Slesinger PA. (2010) Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci, 11 (5): 301-15. [PMID:20389305]
35. Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY. (2002) Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron, 33 (5): 715-29. [PMID:11879649]
36. Nishida M, Cadene M, Chait BT, MacKinnon R. (2007) Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J, 26 (17): 4005-15. [PMID:17703190]
37. Nishida M, MacKinnon R. (2002) Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 A resolution. Cell, 111 (7): 957-65. [PMID:12507423]
38. Padgett CL, Lalive AL, Tan KR, Terunuma M, Munoz MB, Pangalos MN, Martínez-Hernández J, Watanabe M, Moss SJ, Luján R et al.. (2012) Methamphetamine-evoked depression of GABA(B) receptor signaling in GABA neurons of the VTA. Neuron, 73 (5): 978-89. [PMID:22405207]
39. Pegan S, Arrabit C, Zhou W, Kwiatkowski W, Collins A, Slesinger PA, Choe S. (2005) Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat Neurosci, 8 (3): 279-87. [PMID:15723059]
40. Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N. (2002) G(alpha)(i) controls the gating of the G protein-activated K(+) channel, GIRK. Neuron, 33 (1): 87-99. [PMID:11779482]
41. Pravetoni M, Wickman K. (2008) Behavioral characterization of mice lacking GIRK/Kir3 channel subunits. Genes Brain Behav, 7 (5): 523-31. [PMID:18194467]
42. Reuveny E, Slesinger PA, Inglese J, Morales JM, Iñiguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. (1994) Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature, 370 (6485): 143-6. [PMID:8022483]
43. Saitoh O, Masuho I, Terakawa I, Nomoto S, Asano T, Kubo Y. (2001) Regulator of G protein signaling 8 (RGS8) requires its NH2 terminus for subcellular localization and acute desensitization of G protein-gated K+ channels. J Biol Chem, 276 (7): 5052-8. [PMID:11087736]
44. Schoots O, Yue KT, MacDonald JF, Hampson DR, Nobrega JN, Dixon LM, Van Tol HH. (1996) Cloning of a G protein-activated inwardly rectifying potassium channel from human cerebellum. Brain Res Mol Brain Res, 39 (1-2): 23-30. [PMID:8804710]
45. Schreibmayer W, Dessauer CW, Vorobiov D, Gilman AG, Lester HA, Davidson N, Dascal N. (1996) Inhibition of an inwardly rectifying K+ channel by G-protein alpha-subunits. Nature, 380 (6575): 624-7. [PMID:8602262]
46. Torrecilla M, Quillinan N, Williams JT, Wickman K. (2008) Pre- and postsynaptic regulation of locus coeruleus neurons after chronic morphine treatment: a study of GIRK-knockout mice. Eur J Neurosci, 28 (3): 618-24. [PMID:18702733]
47. Wickman K, Pu WT, Clapham DE. (2002) Structural characterization of the mouse Girk genes. Gene, 284 (1-2): 241-50. [PMID:11891065]
48. Wickman KD, Iñiguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. (1994) Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature, 368 (6468): 255-7. [PMID:8145826]
49. Wieting JM, Vadukoot AK, Sharma S, Abney KK, Bridges TM, Daniels JS, Morrison RD, Wickman K, Weaver CD, Hopkins CR. (2017) Discovery and Characterization of 1H-Pyrazol-5-yl-2-phenylacetamides as Novel, Non-Urea-Containing GIRK1/2 Potassium Channel Activators. ACS Chem Neurosci, 8 (9): 1873-1879. [PMID:28697302]