
GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Ghrelin receptor: Introduction
Introduction to the ghrelin receptor
The ghrelin receptor (ghrelinR), previously known as the growth hormone secretagogue receptor 1a, is the receptor for the anabolic hormone ghrelin. This hormone is involved in growth hormone (GH) secretion, appetite regulation, fat accumulation and energy expenditure. In addition it can also modulate behaviour and mood [12,50].
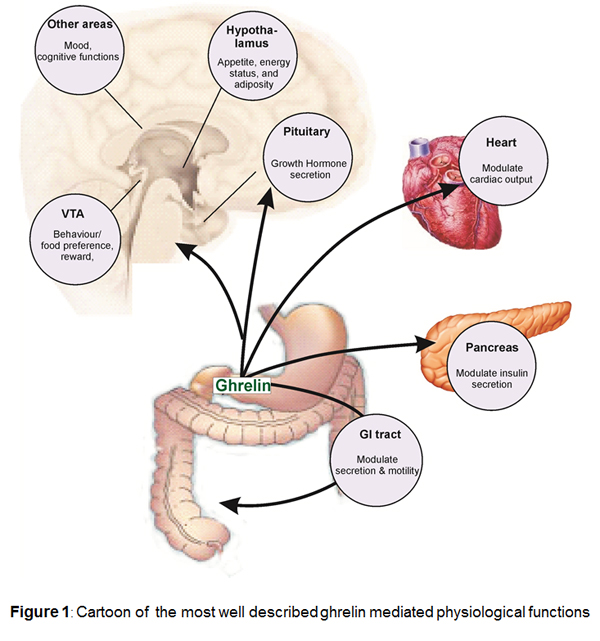
Background
The receptor was cloned by Howard et al. in 1996 from the pituitary and hypothalamus of humans and swine [24], and was shown to be the target of growth hormone secetagogues, a class of peptide and non-peptide compounds leading to growth hormone (GH) release from the anterior pituitary. Nucleotide sequence analysis revealed 2 types of cDNAs, apparently derived from the same gene, which the authors referred to as GHS-R1a and GHS-R1b [24]. The human full-length type, GHS-R1a cDNA encodes the predicted polypeptide of 366 amino acids with 7 transmembrane domains and is the subject of this review. Type Ib is predicted to encode a truncated polypeptide of 289 amino acids with only 5 transmembrane (1-5) domains. The function, if any, is not yet known.
In 1999 the GHS-R1a receptor was paired with ghrelin, using Chinese hamster ovary (CHO) cells expressing the rat GHS-R gene and proposed as the cognate endogenous ligand. Ghrelin is a 28 amino acid peptide originally isolated from rat stomach and is cleaved from a 117 amino acid precursor. The human ghrelin cDNA encodes a prepropeptide with 83% sequence identity to rat prepro-ghrelin. The sequence of the mature rat ghrelin peptide differs by two amino acids from that of the human sequence [29].
Alternative splicing of the ghrelin gene transcript can result in the translation of a second biologically active peptide, des–Gln14-ghrelin [23]. Both peptides have a unique post-translational modification, octanoylation of Ser3, which is essential for the binding to receptors in hypothalamus and pituitary and stimulating the release of growth hormone from the pituitary. However, there is evidence that this modification may not be essential for some of the peripheral effects of ghrelin. For example, Des-octanoyl ghrelin may have cardiovascular effects [2,49]. Antiproliferative actions of ghrelin and growth hormone secretagogues have been observed in breast carcinoma cells not expressing GHS-R mRNA [4-5].
Ghrelin, is thought to be predominantly secreted from X/A like cells within the gastric mucosa [29] and may be the source of the majority of circulating plasma ghrelin [1] although minor sources of ghrelin-like immunoreactivity have been detected in neurones from human pituitary and hypothalamic nuclei [31], rat and human placenta [15] and islet cells in human neonatal pancreas [51]. Early studies in humans and rats showed that ghrelin potently stimulates release of growth hormone from the anterior pituitary. Ghrelin is thought to act on GRLN receptors present on pituitary somatotrophs, and secondly, ghrelin binds to GRLN receptors on growth hormone releasing hormone (GHRH) positive cells in the hypothalamus triggering GHRH liberation. Ghrelin therefore is believed to be involved in the regulation of GH secretion together with the GH liberator GHRH and the GH inhibitor somatostatin.
Physiological functions
Ghrelin stimulates gastric acid secretion and motility. Central and peripheral administration of ghrelin to animals increases food intake leading to weight gain and reduced fat utilization suggesting that the peptide (with several other peptides) may have significant effects on appetite and energy. In a number of species including humans, circulating ghrelin levels significantly increase during fasting and decrease as a response to food intake. This regulatory mechanism of ghrelin secretion is believed to be mediated via cholinergic pathways connecting the gastrointestinal tract with the brain. At the same time ghrelin levels are low in obese and high in lean individuals, suggesting, that ghrelin is not only important for the acute regulation of food intake but also plays an important role in the regulation of long term energy homoeostasis. These functions are consistent with the major source of ghrelin in endocrine cells in the upper gastrointestinal tract [8].
Ghrelin has a number of actions in cardiovascular system, consistent with the localization of receptors to cardiovascular tissue. In humans, the peptide is a potent vasodilator in vivo [41] and in vitro. Ghrelin elicits these actions independent of the endothelium, indicating a direct effect on the vascular smooth muscle [52]. In agreement the ghrelin induced vasodilatation in vivo, is not altered by co-administration of the nitric-oxide-synthase inhibitor L-NMMA. Immunoreactive ghrelin has been detected in endothelial cells throughout the human vasculature, suggesting that the peptide may function as is a ubiquitous endothelium derived vasoactive peptide [28].
Ghrelin functions as a vasodilator in humans. Receptors are significantly up regulated in human atherosclerosis suggesting a role in compensating for the increased vasoconstriction in this condition [26,52]. The precise pathophysiological role of ghrelin has not been established. In a rat model of chronic heart failure (CHF) and in human chronic heart failure patients, ghrelin caused a fall in mean arterial blood pressure and had beneficial effects on stroke volume and cardiac output [38-40] however, whether the observed effects of ghrelin are completely or partially mediated via central GH release remains unclear.
Ghrelin receptor mediated signalling
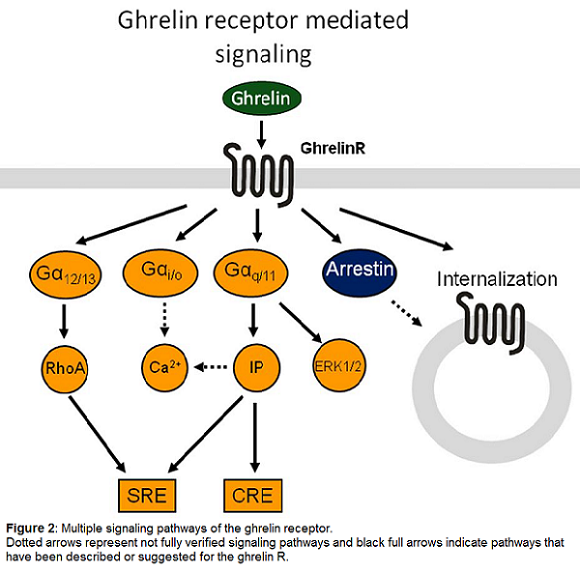
The ghrelinR was originally discovered to induce calcium release during investigation of its growth hormone releasing properties [24]. When the endogenous ligand ghrelin was eventually discovered [29] more thorough studies of the signal transduction properties demonstrated ghrelinR-dependent elevation of the phospholipase C product IP3. Ghrelin has also been reported to activate other downstream signaling pathways such as cAMP response element (CRE) mediated transcription in a dose dependent manner, presumably through Gαq, as CRE can be activated by calcium calmodulin kinase [10,17,19,29]. In addition to the Gαq coupled signaling the ghrelinR couples to Gα12/13 and thereby activates RhoA kinase. The combined actions of Gαq and Gα12/13 are responsible for the majority of the ghrelin induced activation of serum response element (SRE), whereas Gαi coupling is not relevant for this pathway [48], as the signal is unaffected by pertussis toxin. However Gαi/o coupling has been demonstrated in GTPγS assays in model systems [3] as well as in isolated lipid discs [9,33]. Furthermore, ghrelinR activation leads to recruitment of the clathrin adaptor AP2, or β-arrestins, in a manner that is independent of G-protein coupling [9,34]. Stimulation of the ghrelinR also induces ERK1/2 phosphorylation in a dose-dependent manner. This process has been shown to be dependent on protein kinase C (PKC) stimulation and phosphatidylcholine accumulation in a G protein-dependent manner. In contrast, β-arrestin does not play a role in this signaling, since a dominant negative mutant of β-arrestin failed to decrease ERK1/2 phosphorylation [7,35]. This broad variety of signaling possibilities allows development of ligands with functionally biased signaling properties.
Based on structural similarities the closest homolog of the ghrelinR is the motilin receptor, which also has a ligand very similar to ghrelin [6,43]. These two receptors together with the neurotensin, neuromedin U and the orphan receptor GPR39 define a small subfamily of 7TM receptors
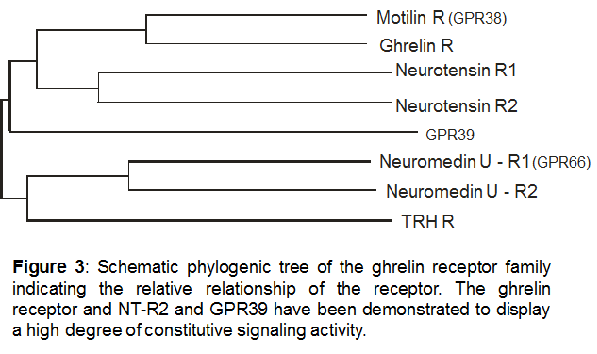
All members of this receptor family have an intron in the coding region between transmembrane regions five and six. For the ghrelin receptor two different splice variants have been identified: the functional receptor, GhrelinR (1a), which has 7 transmembrane helices (GenBank Accession No U60179) and another version, called Ghrelin-R1b, where splicing does not successfully occur and is truncated after the fifth transmembrane region (GenBank Accession U60181).
Unusually, ligand binding is not required for activation of ghrelinR, GPR39 and NTR2. In the absence of any ligand, the ghrelinR signals with almost 50 percent constitutive activity as measured in IP3 accumulation assays and the same level of constitutive signaling has been observed for the NTR2, whereas a low level of constitutive signaling has been observed for the GPR39. This property has for the ghrelinR been demonstrated for several different signaling pathways including SRE and CREB mediated transcriptional activity [19]. Recruitment of arrestin has also been shown to occur in a ligand independent manner in heterologous expression systems, whereas in isolated lipid discs the arrestin recruitment is ligand dependent [33-34]. Internalization of the ghrelinR can occur in a ligand independent manner, dependent on receptor constitutive activity and domains within the C-terminal tail [16]. The recent demonstration of ligand independent AP2 recruitment to the ghrelinR may therefore contribute to these basal receptor internalization properties [9]. Interestingly however, some signaling pathways, such as ERK phosphorylation and Gαi coupling, do require receptor activation by exogenous agonists [9,19]. Recent studies show that the constitutive activity of the ghrelinR is an intrinsic property of the ghrelinR as the receptor embedded in lipid discs induces inositol phosphate accumulation and GTPγS binding; these signaling effects can be reduced by addition of an inverse agonist or increased by agonists [22].
Ligand development for the ghrelinR was initially focused on agonists to increase growth hormone secretion. Several high potency efficacious agonists, based on either peptide or non-peptide scaffolds, were studied in clinical trials, when the appetite promoting effects of the ghrelin system was revealed and ligands to block the function of ghrelin subsequently became the major focus. The substance P analogue ([D-Arg1, D-Phe5, D-Trp7,9, Leu11]-substance P), was the first antagonist and inverse agonist to be identified for the ghrelinR. Since then, several other ligands for the ghrelinR have been discovered. Based on a conserved motif wFwLL of the substance P analogue, a series of inverse agonists has been developed [17]. Small molecule antagonists have more recently been discovered for the ghrelinR in an attempt to develop a treatment for obesity, however the molecular pharmacological properties of these compounds remain to be fully characterized [42,45,53].
Ligand interaction with the ghrelinR has also been studied for several agonist compounds and for a few peptide-based inverse agonists, by mutation studies in combination with computational chemistry. The most important ligand interaction site described in the ghrelinR is a glutamic acid in the extracellular part of TM III. This is pivotal for binding and function of both ghrelin and almost all ghrelinR ligands. Furthermore, mutations of aromatic and positively charged residues in TM VI affect the potency of ghrelin significantly [13,18,20]. Apart from these residues, no other substitutions in the binding pocket affect ghrelin induced activation, indicating that ghrelin only makes a few key interactions within the centre of the binding pocket of the receptor in addition to potential interactions in the extracellular loops [18,32]. However, ghrelinR constitutive activity is diminished by substitutions in several other receptor domains, including substitutions of aromatic and charged residues in the extracellular part of TMIII, TMVI and VII [14,19]. These studies demonstrate the importance of a hydrophobic cluster connecting the extracellular parts of TM VI and VII for the activation of the receptor. Finally, inverse agonists generally require interactions deep in the transmembrane binding pocket, compared to the interactions made by agonists occuring more superficially towards the extracellular ends of the TM domains [18,21].
Biased signaling properties
A classical biased agonist, wFw-Isn, has been discovered for the ghrelinR which favours signaling through one G-protein compared to another [48]. This biased agonist contains the core binding wFw motif, and is linked to a synthetic amino acid isonipecotic acid (Isn) at the C-terminus. Mutational studies and computational docking studies reveal that the interaction pattern of wFw-Isn is different from all other described peptide and non-peptide ghrelinR ligands. Importantly, the activity of this agonist is not dependent on the highly conserved negatively charged glutamic acid in TMIII that serves as the receptor anchoring point for all other ligands described for the ghrelinR. This ligand induces IP3 accumulation and ERK1/2 phosphorylation, but it is unable to activate the Gα12/13 coupled, SRE-induced transcriptional activity. These data suggest that wFw-Isn is a biased ligand that favours pathways such as Gαq and ERK1/2 phosphorylation over Gα12/13coupling. In support of this notion, wFw-Isn does not activate RhoA in a pituitary cell line naturally expressing the ghrelinR, unlike ghrelin itself [48]. Interestingly, wFw-Isn administrated i.c.v. to rats was unable to stimulate food intake, which is one of the most solid and reproducible effects of ghrelinR stimulation. These data suggest that it is possible to rationally develop biased agonists based on deep understanding of ligand receptor interaction. In addition the hexapeptide ligand, KwFwLL, has been identified, unusually, as a biased inverse agonist for the ghrelinR. It is a potent inverse agonist when measuring IP3 accumulation with an EC50 value of 32nM, however when measuring SRE transcriptional activity it is a very low potency inverse agonist that only induces a weak decrease in the signaling at 1µM concentration [21]. This observation is most likely due to biased inverse agonist signaling, favouring inhibition of the Gαq coupled pathway compared to coupling to Gα12/13.
The above mentioned examples of biased ligands for the ghrelinR were characterized in vitro, predominantly in heterologous expression systems. However, recently the ghrelinR was reconstituted in lipid discs. Receptor activation was measured by monitoring conformational changes via fluorescent probes in the intracellular and in the extracellular domains. Under these conditions the ghrelin receptor was able to couple to Gαi in addition to the previously mentioned signaling pathways. In this system a biased agonist, JMV3018, was demonstrated to couple to Gαq to the same extent as ghrelin but was unable to couple to Gαi or recruit β-arrestin [33].
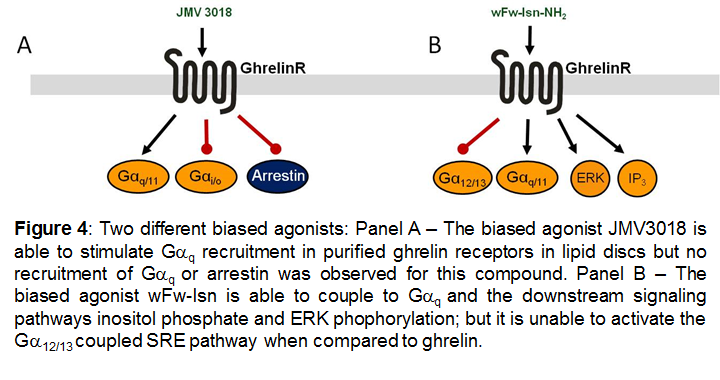
Figures 1, 2 and 4 are reproduced with the author's permission from [47].
Dimerization
The ghrelinR has been shown to dimerize both with other 7TM receptors as heterodimers and with itself as a homodimer. The interface responsible for the dimerization has not been studied for this receptor family. However, it has been demonstrated that the dopamine D1 receptor [25], dopamine D2 receptor [27], melanocortin MC3 receptor [44] and the serotonin 5-HT2c receptor [46], are co-expressed with the ghrelinR under physiological conditions. Importantly it has been shown that co-expression of the ghrelinR with either the MC3, the D1 or the 5HT2c receptor leads to decreased ghrelin mediated signaling in heterologlous expression systems. In addition α-MSH signaling is enhanced by co-expression of the ghrelin- and the MC3 receptors.
Summary
The ghrelinR is a 7TM receptor with metabolic physiological functions. Most importantly it is involved in appetite regulation, energy homeostasis and fat accumulation as well as mood regulation, cognitive functions and reward-related food behaviour. An official nomenclature that briefly highlights the significance of ghrelin and its receptor has been published in Pharmacological Reviews [11]. For more detailed information on function see reviews [30,36-37,50].
The receptor couples to many different signaling pathways, that in an integrated manner determine the cellular and physiological function induced by ghrelin. Increased molecular understanding of the biased signaling properties and the importance of dimerization with other 7TM receptors may reveal a better understanding of selective ghrelin-induced activation of specific physiological functions.
References
1. Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S et al.. (2001) Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab, 86 (10): 4753-8. [PMID:11600536]
2. Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F et al.. (2002) Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol, 159 (6): 1029-37. [PMID:12486113]
3. Bennett KA, Langmead CJ, Wise A, Milligan G. (2009) Growth hormone secretagogues and growth hormone releasing peptides act as orthosteric super-agonists but not allosteric regulators for activation of the G protein Galpha(o1) by the Ghrelin receptor. Mol Pharmacol, 76 (4): 802-11. [PMID:19625579]
4. Cassoni P, Ghé C, Marrocco T, Tarabra E, Allia E, Catapano F, Deghenghi R, Ghigo E, Papotti M, Muccioli G. (2004) Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. Eur J Endocrinol, 150 (2): 173-84. [PMID:14763915]
5. Cassoni P, Papotti M, Ghè C, Catapano F, Sapino A, Graziani A, Deghenghi R, Reissmann T, Ghigo E, Muccioli G. (2001) Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab, 86 (4): 1738-45. [PMID:11297611]
6. Chen CY, Tsai CY. (2012) Ghrelin and motilin in the gastrointestinal system. Curr Pharm Des, 18 (31): 4755-65. [PMID:22632857]
7. Chu KM, Chow KB, Leung PK, Lau PN, Chan CB, Cheng CH, Wise H. (2007) Over-expression of the truncated ghrelin receptor polypeptide attenuates the constitutive activation of phosphatidylinositol-specific phospholipase C by ghrelin receptors but has no effect on ghrelin-stimulated extracellular signal-regulated kinase 1/2 activity. Int J Biochem Cell Biol, 39 (4): 752-64. [PMID:17169600]
8. Cummings DE, Foster-Schubert KE, Overduin J. (2005) Ghrelin and energy balance: focus on current controversies. Curr Drug Targets, 6 (2): 153-69. [PMID:15777186]
9. Damian M, Marie J, Leyris JP, Fehrentz JA, Verdié P, Martinez J, Banères JL, Mary S. (2012) High constitutive activity is an intrinsic feature of ghrelin receptor protein: a study with a functional monomeric GHS-R1a receptor reconstituted in lipid discs. J Biol Chem, 287 (6): 3630-41. [PMID:22117076]
10. Dash PK, Karl KA, Colicos MA, Prywes R, Kandel ER. (1991) cAMP response element-binding protein is activated by Ca2+/calmodulin- as well as cAMP-dependent protein kinase. Proc Natl Acad Sci USA, 88 (11): 5061-5. [PMID:1647024]
11. Davenport AP, Bonner TI, Foord SM, Harmar AJ, Neubig RR, Pin JP, Spedding M, Kojima M, Kangawa K. (2005) International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol Rev, 57 (4): 541-6. [PMID:16382107]
12. Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. (2011) The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol, 340 (1): 80-7. [PMID:21354264]
13. Feighner SD, Howard AD, Prendergast K, Palyha OC, Hreniuk DL, Nargund R, Underwood D, Tata JR, Dean DC, Tan CP et al.. (1998) Structural requirements for the activation of the human growth hormone secretagogue receptor by peptide and nonpeptide secretagogues. Mol Endocrinol, 12 (1): 137-45. [PMID:9440817]
14. Gozé C, Bergé G, M'Kadmi C, Floquet N, Gagne D, Galleyrand JC, Fehrentz JA, Martinez J. (2010) Involvement of tryptophan W276 and of two surrounding amino acid residues in the high constitutive activity of the ghrelin receptor GHS-R1a. Eur J Pharmacol, 643 (2-3): 153-61. [PMID:20599926]
15. Gualillo O, Caminos J, Blanco M, Garcìa-Caballero T, Kojima M, Kangawa K, Dieguez C, Casanueva F. (2001) Ghrelin, a novel placental-derived hormone. Endocrinology, 142 (2): 788-94. [PMID:11159851]
16. Holliday ND, Holst B, Rodionova EA, Schwartz TW, Cox HM. (2007) Importance of constitutive activity and arrestin-independent mechanisms for intracellular trafficking of the ghrelin receptor. Mol Endocrinol, 21 (12): 3100-12. [PMID:17717076]
17. Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. (2003) High constitutive signaling of the ghrelin receptor--identification of a potent inverse agonist. Mol Endocrinol, 17 (11): 2201-10. [PMID:12907757]
18. Holst B, Frimurer TM, Mokrosinski J, Halkjaer T, Cullberg KB, Underwood CR, Schwartz TW. (2009) Overlapping binding site for the endogenous agonist, small-molecule agonists, and ago-allosteric modulators on the ghrelin receptor. Mol Pharmacol, 75 (1): 44-59. [PMID:18923064]
19. Holst B, Holliday ND, Bach A, Elling CE, Cox HM, Schwartz TW. (2004) Common structural basis for constitutive activity of the ghrelin receptor family. J Biol Chem, 279 (51): 53806-17. [PMID:15383539]
20. Holst B, Lang M, Brandt E, Bach A, Howard A, Frimurer TM, Beck-Sickinger A, Schwartz TW. (2006) Ghrelin receptor inverse agonists: identification of an active peptide core and its interaction epitopes on the receptor. Mol Pharmacol, 70 (3): 936-46. [PMID:16798937]
21. Holst B, Mokrosinski J, Lang M, Brandt E, Nygaard R, Frimurer TM, Beck-Sickinger AG, Schwartz TW. (2007) Identification of an efficacy switch region in the ghrelin receptor responsible for interchange between agonism and inverse agonism. J Biol Chem, 282 (21): 15799-811. [PMID:17371869]
22. Holst B, Schwartz TW. (2004) Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacol Sci, 25 (3): 113-7. [PMID:15058279]
23. Hosoda H, Kojima M, Matsuo H, Kangawa K. (2000) Purification and characterization of rat des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J Biol Chem, 275 (29): 21995-2000. [PMID:10801861]
24. Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J et al.. (1996) A receptor in pituitary and hypothalamus that functions in growth hormone release. Science, 273 (5277): 974-7. [PMID:8688086]
25. Jiang H, Betancourt L, Smith RG. (2006) Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol, 20 (8): 1772-85. [PMID:16601073]
26. Katugampola SD, Pallikaros Z, Davenport AP. (2001) [125I-His(9)]-ghrelin, a novel radioligand for localizing GHS orphan receptors in human and rat tissue: up-regulation of receptors with athersclerosis. Br J Pharmacol, 134 (1): 143-9. [PMID:11522606]
27. Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. (2012) Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron, 73 (2): 317-32. [PMID:22284186]
28. Kleinz MJ, Kuc RE, Maguire JJ, Davenport AP. Endogenous vasodilator ghrelin and its growth hormone secretagogue receptor 1a (GHS-R1a) immunocytochemically localize to endothelial cells within the human vasculature. Accessed on 05/05/2015. Modified on 05/05/2015. BPS pA2 online abstracts, http://www.pa2online.org/scripts/relatedVol1Issue3.asp
29. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature, 402 (6762): 656-60. [PMID:10604470]
30. Kojima M, Kangawa K. (2005) Ghrelin: structure and function. Physiol Rev, 85 (2): 495-522. [PMID:15788704]
31. Korbonits M, Kojima M, Kangawa K, Grossman AB. (2001) Presence of ghrelin in normal and adenomatous human pituitary. Endocrine, 14 (1): 101-4. [PMID:11322490]
32. Malcolm JA, Sutherland DC. (1991) HIV and nutrition. Lancet, 338 (8769): 760. [PMID:1679893]
33. Mary S, Damian M, Louet M, Floquet N, Fehrentz JA, Marie J, Martinez J, Banères JL. (2012) Ligands and signaling proteins govern the conformational landscape explored by a G protein-coupled receptor. Proc Natl Acad Sci USA, 109 (21): 8304-9. [PMID:22573814]
34. Mokrosiński J, Frimurer TM, Sivertsen B, Schwartz TW, Holst B. (2012) Modulation of constitutive activity and signaling bias of the ghrelin receptor by conformational constraint in the second extracellular loop. J Biol Chem, 287 (40): 33488-502. [PMID:22846991]
35. Mousseaux D, Le Gallic L, Ryan J, Oiry C, Gagne D, Fehrentz JA, Galleyrand JC, Martinez J. (2006) Regulation of ERK1/2 activity by ghrelin-activated growth hormone secretagogue receptor 1A involves a PLC/PKCvarepsilon pathway. Br J Pharmacol, 148 (3): 350-65. [PMID:16582936]
36. Nagaya N, Kangawa K. (2003) Ghrelin improves left ventricular dysfunction and cardiac cachexia in heart failure. Curr Opin Pharmacol, 3 (2): 146-51. [PMID:12681236]
37. Nagaya N, Kangawa K. (2003) Ghrelin, a novel growth hormone-releasing peptide, in the treatment of chronic heart failure. Regul Pept, 114 (2-3): 71-7. [PMID:12832093]
38. Nagaya N, Kojima M, Uematsu M, Yamagishi M, Hosoda H, Oya H, Hayashi Y, Kangawa K. (2001) Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol Regul Integr Comp Physiol, 280 (5): R1483-7. [PMID:11294772]
39. Nagaya N, Miyatake K, Uematsu M, Oya H, Shimizu W, Hosoda H, Kojima M, Nakanishi N, Mori H, Kangawa K. (2001) Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J Clin Endocrinol Metab, 86 (12): 5854-9. [PMID:11739451]
40. Nagaya N, Uematsu M, Kojima M, Ikeda Y, Yoshihara F, Shimizu W, Hosoda H, Hirota Y, Ishida H, Mori H et al.. (2001) Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation, 104 (12): 1430-5. [PMID:11560861]
41. Okumura H, Nagaya N, Enomoto M, Nakagawa E, Oya H, Kangawa K. (2002) Vasodilatory effect of ghrelin, an endogenous peptide from the stomach. J Cardiovasc Pharmacol, 39: 779-783. [PMID:12021570]
42. Palus S, Schur R, Akashi YJ, Bockmeyer B, Datta R, Halem H, Dong J, Culler MD, Adams V, Anker SD et al.. (2011) Ghrelin and its analogues, BIM-28131 and BIM-28125, improve body weight and regulate the expression of MuRF-1 and MAFbx in a rat heart failure model. PLoS ONE, 6 (11): e26865. [PMID:22102869]
43. Poitras P, Peeters TL. (2008) Motilin. Curr Opin Endocrinol Diabetes Obes, 15 (1): 54-7. [PMID:18185063]
44. Rediger A, Piechowski CL, Habegger K, Grüters A, Krude H, Tschöp MH, Kleinau G, Biebermann H. (2012) MC4R dimerization in the paraventricular nucleus and GHSR/MC3R heterodimerization in the arcuate nucleus: is there relevance for body weight regulation?. Neuroendocrinology, 95 (4): 277-88. [PMID:22327910]
45. Rudolph J, Esler WP, O'connor S, Coish PD, Wickens PL, Brands M, Bierer DE, Bloomquist BT, Bondar G, Chen L et al.. (2007) Quinazolinone derivatives as orally available ghrelin receptor antagonists for the treatment of diabetes and obesity. J Med Chem, 50 (21): 5202-16. [PMID:17887659]
46. Schellekens H, van Oeffelen WE, Dinan TG, Cryan JF. (2013) Promiscuous dimerization of the growth hormone secretagogue receptor (GHS-R1a) attenuates ghrelin-mediated signaling. J Biol Chem, 288 (1): 181-91. [PMID:23161547]
47. Sivertsen B, Holliday N, Madsen AN, Holst B. (2013) Functionally biased signalling properties of 7TM receptors - opportunities for drug development for the ghrelin receptor. Br J Pharmacol, 170 (7): 1349-62. [PMID:24032557]
48. Sivertsen B, Lang M, Frimurer TM, Holliday ND, Bach A, Els S, Engelstoft MS, Petersen PS, Madsen AN, Schwartz TW et al.. (2011) Unique interaction pattern for a functionally biased ghrelin receptor agonist. J Biol Chem, 286 (23): 20845-60. [PMID:21402696]
49. Tsubota Y, Owada-Makabe K, Yukawa K, Maeda M. (2005) Hypotensive effect of des-acyl ghrelin at nucleus tractus solitarii of rat. Neuroreport, 16 (2): 163-6. [PMID:15671869]
50. van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. (2004) Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev, 25 (3): 426-57. [PMID:15180951]
51. Wierup N, Svensson H, Mulder H, Sundler F. (2002) The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept, 107 (1-3): 63-9. [PMID:12137967]
52. Wiley KE, Davenport AP. (2002) Comparison of vasodilators in human internal mammary artery: ghrelin is a potent physiological antagonist of endothelin-1. Br J Pharmacol, 136 (8): 1146-52. [PMID:12163347]
53. Xin Z, Serby MD, Zhao H, Kosogof C, Szczepankiewicz BG, Liu M, Liu B, Hutchins CW, Sarris KA, Hoff ED et al.. (2006) Discovery and pharmacological evaluation of growth hormone secretagogue receptor antagonists. J Med Chem, 49 (15): 4459-69. [PMID:16854051]







