
GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Leukotriene receptors: Introduction
Introduction
The endogenous ligands for the leukotriene, lipoxin and oxoeicosanoid receptors are bioactive products produced by the action of the lipoxygenase family of enzymes, as depicted in Fig 1 [1,4]. The formation of leukotrienes by 5-lipoxygenase from acid arachidonic acid starts with the epoxide intermediate LTA4, which serves as precursor for leukotriene synthesis (Fig. 1). Subsequent metabolism through the enzyme LTA4 hydrolase leads to the formation of LTB4, which is the ligand for the BLT receptors. Alternatively, the conjugation of LTA4 with glutathione yields the cysteinyl-leukotrienes (Fig 1) acting on CysLT receptors (Fig 1). The leukotriene receptor class also includes receptors for other mediators derived from arachidonic acid through lipoxygenase metabolism, namely the ALX/FPR2 receptor for lipoxin A4 [10], and the OXE receptor for 5-Oxo-ETE [2], as indicated in Fig 1.
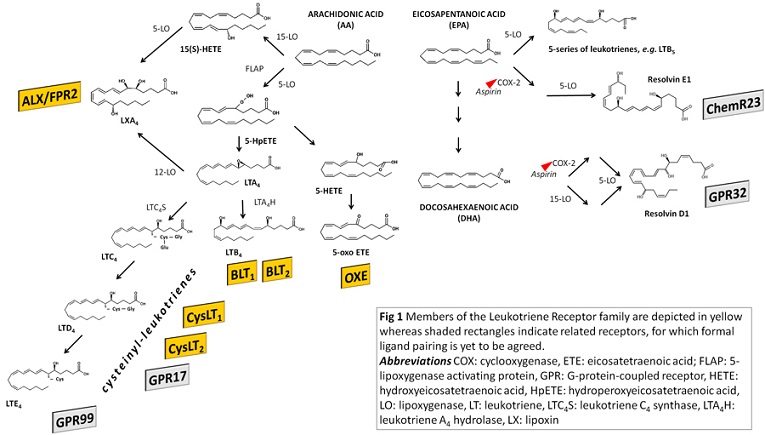
BLT receptors
The dihydroxy-leukotriene, leukotriene B4 (LTB4) stimulates neutrophil chemotaxis and secretion but may also affect immunomodulation through the activation of several leukocyte populations [1,4]. In addition, receptors for LTB4 are expressed on non-myeloid cells, such as vascular smooth muscle and endothelial cells [3]. Chemotaxis, the principal effects of LTB4 and related dihydroxy-acids on leukocytes, occurs via activation of BLT1 receptors [58]. The human BLT1 receptor is the high affinity LTB4 receptor, consisting of 352 amino acids and encoded by a gene located on chromosome 14q11.2-q12 [58]. Transgenic overexpression of the human BLT1 receptor in mice increased the inflammatory response to LTB4 [9], whereas genetic BLT1 receptor disruption decreased leukocyte chemotaxis, and protected against disease development in several different animal models [1,4]. Although the LTB4-induced interaction with the BLT1 receptor corresponded with several effects observed in those target cells, initial studies had revealed both high and lower affinity binding sites for LTB4 specifically in human granulocytes. The molecular explanation for the latter finding was provided in 2000, when a gene with high sequence similarity to BLT1 receptor was identified, and encoded a low affinity LTB4 receptor which has subsequently been denoted BLT2 [1,4,60]. Although LTB4 appears to be the sole full agonist for the BLT1 receptor, several lipoxygenase-products in addition to LTB4 have been identified as ligands for the human BLT2 receptor. These include 12(S)-HETE, 12(S)-HpETE, and 15(S)-HETE [59]. Furthermore, the thromboxane synthase product 12-HHT formed in activated blood platelets and macrophages from prostaglandin H2, is also a natural ligand for the BLT2 receptor [39].
The inflammatory LTB4 signaling through the BLT1 and BLT2 receptors has been implicated in several disease, such as for example bronchial asthma [55], rheumatoid arthritis [27,51], atherosclerosis [3,5], abdominal aortic aneurysms [23], bone metabolism [22], multiple sclerosis [26], and cancer [61].
CysLT receptors
The cysteinyl-leukotrienes (LTC4, LTD4 and LTE4; cys-LTs) are known to be potent smooth muscle contractile agents [14]. However, these mediators have also been reported to cause plasma exudation [12], recruit eosinophils, provoke cardiodepression, induce cell proliferation and increase mucous production. Over the years, a large number of selective antagonists for CysLT receptors have been developed. These antagonists allowed to initially define the two broad subgroups of CysLT receptors, those that were blocked by these antagonists (CysLT1) and those that were resistant to blockade (CysLT2) [28].
The CysLT1 and CysLT2 receptors and are glycosylated G-protein coupled receptors with 337 and 346 amino acids, respectively [21,30]. The CysLT1 receptor is expressed in many human tissues including lung, peripheral blood leukocytes, spleen and placenta [1,4]. Despite some overlapping with the CysLT1 receptor, the CysLT2 receptor exhibits a distinctive expression pattern in for example the heart, brain, and adrenal glands [4,7]. In cells transfected with the CysLT1 recombinant receptor, LTD4 was demonstrated to be more potent than LTC4 and LTE4 [1,4]. In contrast, when similar experiments were performed with the CysLT2 recombinant receptor the agonist rank order potency was LTD4=LTC4 with LTE4 less potent [1,4]. The classical CysLT1 antagonists blocked the cysteinyl-leukotriene-induced calcium mobilization in the CysLT1 receptor transfected cells whereas in cells transfected with the CysLT2 receptor, the ligand activation of calcium mobilization was not blocked by these antagonists. Furthermore, the BAY u9773 compound was shown to be a CysLT2 subtype selective agonist for calcium mobilization in transfected cells [1,4]. Although CysLT receptor signaling originate from plasma membrane expression, recent studies have also demonstrated a nuclear/perinuclear expression of CysLT1 receptors in different cell types [15,34,36].
Cysteinyl-leukotriene signaling through the CysLT1 receptor has been mainly explored in bronchial asthma and allergic rhinitis, for which CysLT1 receptor antagonist are presently used clinically [45]. In addition, observational studies have suggested beneficial effects of this class of drugs for the prevention of myocardial infarction and stroke [24], and experimental studies have shown a role in aortic valve stenosis [34]. The abundant expression of the CysLT2 receptor in endothelial cells of some vascular beds, has also been implied in myocardial damage induced by ischemia/reperfusion [35]. A role for CysLT1 and CysLT2 activation in various cancers has also been demonstrated [4].
There is also evidence in the literature for additional CysLT receptor subtypes, derived from functional in vitro studies [6,29,48,52,56], radioligand binding [8,46-47] and in mice lacking both CysLT1 and CysLT2 receptors [32].
LTE4 has been suggested to signal through P2Y12 receptors in some studies [19,37,40], although not consequently replicated in all settings [18]. In support of common purinergic and leukotriene signaling, the orphan GPR17 (Fig 1) has been postulated to be activated by both cysteinyl-leukotrienes and nucleotides [11], and to function as a negative regulator for LTD4-induced CysLT1 receptor mediated responses [31]. The formal pairing of GPR17 as a dual receptor for cysteinyl-leukotrienes and nucleotides is yet to be agreed [13].
Recent evidence point to yet another receptor for cysteinyl-leukotrienes, namely GPR99 [25] (Fig 1), which was initially recognized as the oxoglutarate receptor (OXGR1) based on its binding of 2-oxoglutarate (α-ketoglutarate) [13]. Cells transfected with the human GPR99 exhibit both functional and binding responses to LTE4, and GPR99 deletion in mice abrogates LTE4-induced vascular leakage [25]. Further investigations are presently necessary to distinguish the preferred endogenous ligands for GPR99.
ALX/FPR2 receptor
The dual lipoxygenation of arachidonic acid by either the 15- and 5-lipoxygenase or the 5- and 12-lipoxygenase produced eicosanoids known as lipoxins, as indicated in Fig 1 [10]. Lipoxins are inhibitory or anti-inflammatory mediators which act as a "stop signal" during inflammatory reactions [7,49]. At the molecular level the lipoxin receptor was the first recognized non-prostanoid eicosanoid G-protein coupled receptor [16-17]. This receptor is composed of 351 amino acids and the gene located to the X chromosome (19q13.3). Abundant receptor expressed is found in the lung, peripheral blood leukocytes, spleen, and lesser amounts in the heart, placenta and liver [10]. Since this receptor had high sequence homology (70%) to the formyl peptide receptor (FPR), it was initially designated as formyl peptide like receptor-1 (FPRL-1). However, although a number of peptides activate the receptor, LXA4 is the most potent native endogenous ligand. Therefore the nomenclature recommended for this receptor is FPR2/ALX, and ALX/FPR2 when the lipoxin-binding property is of primary concern [57].
OXE receptor
Oxoeicosanoids are a family of biologically active arachidonic acid derivatives that have been intimately associated with cellular migration [2]. 5-Oxo-ETE is formed by the oxidation of 5S-HETE by 5-hydroxyeicosanoid dehydrogenase, a highly selective NADP+-dependent enzyme (Fig 1) [44]. This mediator is a potent chemoattractant for human neutrophils, eosinophils, monocytes, and basophils [41-43].
Biological responses to 5-oxo-ETE are mediated by the OXE receptor, which is encoded by the OXER1 gene. This receptor was identified independently by three groups and was previously known as TG1019, R527 and GPCR48. Consistent with the biological activities of 5-oxo-ETE, the OXE receptor is highly expressed in human eosinophils ≈ basophils > neutrophils > macrophages [2] and is also expressed in a variety of cancer cell lines [38].
The OXE receptor has an amino acid composition of 423 and a gene localized to chromosome 2p21. The OXE receptor shares 23.2% and 25.3% identity with CysLT1 and CysLT2 respectively [2].
Since a major target of 5-oxo-ETE is the eosinophil, the OXE receptor could play an important role in eosinophilic disorders such as asthma and allergic rhinitis [33]. In addition, the OXE receptor could also be an important drug target in cancer, as it appears to play a role in cancer cell proliferation and its downregulation with siRNA has an antiproliferative effect [54]. The availability of OXE receptor antagonists, such as the recently reported indole derivative 5-(6-chloro-2-hexyl-1H-indol-1-yl)-5-oxo-valeric acid, will facilitate future investigations of OXE receptor signaling in different disease models [20].
Resolvin receptors
Besides the 20:4, n-6 fatty acid arachidonic acid, also omega-3 essential polyunsaturated fatty acids, such as eicosapentanoic acid (EPA; 20:5, n-3) and docosahexaenoic acid (DHA; 22:6, n-3), are metabolized by lipoxygenases in human cells (Fig 1). For example, when metabolized by 5-lipoxygenase, EPA generates leukotrienes of the 5-series (e.g. LTB5, see Fig 1) which are not biologically active but compete with leukotriene binding to the leukotriene receptors, suggesting that lipoxygenase metabolites of omega-3 fatty acids may act as inhibitors of inflammation [53]. Furthermore, EPA and DHA can enter into the lipoxygenase metabolism and lead to the biosynthesis of either E-series (for EPA-derived), or D-series (for DHA-derived) of Resolvins. These omega-3-derived mediators have been characterized as mediators of inflammation resolution. Although not yet formally classified as lipid mediator receptors, several lines of recent evidence point to GPR32 as the receptor for resolvin D1 and ChemR23 (or CMKLR1) as the receptor for resolvin E1 (Fig 1) [7,50]. The formal pairing of these receptors with their cognate ligands is however yet to be agreed [13].
References
1. Brink C, Dahlén SE, Drazen J, Evans JF, Hay DW, Nicosia S, Serhan CN, Shimizu T, Yokomizo T. (2003) International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev, 55 (1): 195-227. [PMID:12615958]
2. Brink C, Dahlén SE, Drazen J, Evans JF, Hay DW, Rovati GE, Serhan CN, Shimizu T, Yokomizo T. (2004) International Union of Pharmacology XLIV. Nomenclature for the oxoeicosanoid receptor. Pharmacol Rev, 56 (1): 149-57. [PMID:15001665]
3. Bäck M, Bu DX, Bränström R, Sheikine Y, Yan ZQ, Hansson GK. (2005) Leukotriene B4 signaling through NF-kappaB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc Natl Acad Sci USA, 102 (48): 17501-6. [PMID:16293697]
4. Bäck M, Dahlén SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE. (2011) International Union of Basic and Clinical Pharmacology. LXXXIV: leukotriene receptor nomenclature, distribution, and pathophysiological functions. Pharmacol Rev, 63 (3): 539-84. [PMID:21771892]
5. Bäck M, Hansson GK. (2006) Leukotriene receptors in atherosclerosis. Ann Med, 38 (7): 493-502. [PMID:17101540]
6. Bäck M, Norel X, Walch L, Gascard J, Mazmanian G, Dahlén S, Brink C. (2000) Antagonist resistant contractions of the porcine pulmonary artery by cysteinyl-leukotrienes. Eur J Pharmacol, 401 (3): 381-8. [PMID:10936497]
7. Capra V, Bäck M, Barbieri SS, Camera M, Tremoli E, Rovati GE. (2013) Eicosanoids and their drugs in cardiovascular diseases: focus on atherosclerosis and stroke. Med Res Rev, 33 (2): 364-438. [PMID:22434418]
8. Capra V, Nicosia S, Ragnini D, Mezzetti M, Keppler D, Rovati GE. (1998) Identification and characterization of two cysteinyl-leukotriene high affinity binding sites with receptor characteristics in human lung parenchyma. Mol Pharmacol, 53 (4): 750-8. [PMID:9547367]
9. Chiang N, Gronert K, Clish CB, O'Brien JA, Freeman MW, Serhan CN. (1999) Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest, 104 (3): 309-16. [PMID:10430612]
10. Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. (2006) The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev, 58 (3): 463-87. [PMID:16968948]
11. Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP. (2006) The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J, 25 (19): 4615-27. [PMID:16990797]
12. Dahlén SE, Björk J, Hedqvist P, Arfors KE, Hammarström S, Lindgren JA, Samuelsson B. (1981) Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc Natl Acad Sci USA, 78 (6): 3887-91. [PMID:6267608]
13. Davenport AP, Alexander SP, Sharman JL, Pawson AJ, Benson HE, Monaghan AE, Liew WC, Mpamhanga CP, Bonner TI, Neubig RR et al.. (2013) International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol Rev, 65 (3): 967-86. [PMID:23686350]
14. Drazen JM, Austen KF, Lewis RA, Clark DA, Goto G, Marfat A, Corey EJ. (1980) Comparative airway and vascular activities of leukotrienes C-1 and D in vivo and in vitro. Proc Natl Acad Sci USA, 77 (7): 4354-8. [PMID:6933488]
15. Eaton A, Nagy E, Pacault M, Fauconnier J, Bäck M. (2012) Cysteinyl leukotriene signaling through perinuclear CysLT(1) receptors on vascular smooth muscle cells transduces nuclear calcium signaling and alterations of gene expression. J Mol Med, 90 (10): 1223-31. [PMID:22527886]
16. Fiore S, Maddox JF, Perez HD, Serhan CN. (1994) Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med, 180 (1): 253-60. [PMID:8006586]
17. Fiore S, Romano M, Reardon EM, Serhan CN. (1993) Induction of functional lipoxin A4 receptors in HL-60 cells. Blood, 81 (12): 3395-403. [PMID:8389617]
18. Foster HR, Fuerst E, Lee TH, Cousins DJ, Woszczek G. (2013) Characterisation of P2Y(12) receptor responsiveness to cysteinyl leukotrienes. PLoS ONE, 8 (3): e58305. [PMID:23472176]
19. Fredman G, Van Dyke TE, Serhan CN. (2010) Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol, 30 (10): 2005-13. [PMID:20702811]
20. Gore V, Patel P, Chang CT, Sivendran S, Kang N, Ouedraogo YP, Gravel S, Powell WS, Rokach J. (2013) 5-Oxo-ETE receptor antagonists. J Med Chem, 56 (9): 3725-32. [PMID:23581530]
21. Heise CE, O'Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R et al.. (2000) Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem, 275 (39): 30531-6. [PMID:10851239]
22. Hikiji H, Ishii S, Yokomizo T, Takato T, Shimizu T. (2009) A distinctive role of the leukotriene B4 receptor BLT1 in osteoclastic activity during bone loss. Proc Natl Acad Sci USA, 106 (50): 21294-9. [PMID:19965376]
23. Houard X, Ollivier V, Louedec L, Michel JB, Bäck M. (2009) Differential inflammatory activity across human abdominal aortic aneurysms reveals neutrophil-derived leukotriene B4 as a major chemotactic factor released from the intraluminal thrombus. FASEB J, 23 (5): 1376-83. [PMID:19136615]
24. Ingelsson E, Yin L, Bäck M. (2012) Nationwide cohort study of the leukotriene receptor antagonist montelukast and incident or recurrent cardiovascular disease. J Allergy Clin Immunol, 129 (3): 702-707.e2. [PMID:22244598]
25. Kanaoka Y, Maekawa A, Austen KF. (2013) Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J Biol Chem, 288 (16): 10967-72. [PMID:23504326]
26. Kihara Y, Yokomizo T, Kunita A, Morishita Y, Fukayama M, Ishii S, Shimizu T. (2010) The leukotriene B4 receptor, BLT1, is required for the induction of experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun, 394 (3): 673-8. [PMID:20226760]
27. Kim ND, Chou RC, Seung E, Tager AM, Luster AD. (2006) A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med, 203 (4): 829-35. [PMID:16567386]
28. Labat C, Ortiz JL, Norel X, Gorenne I, Verley J, Abram TS, Cuthbert NJ, Tudhope SR, Norman P, Gardiner P et al.. (1992) A second cysteinyl leukotriene receptor in human lung. J Pharmacol Exp Ther, 263 (2): 800-5. [PMID:1331415]
29. Lee TH, Austen KF, Corey EJ, Drazen JM. (1984) Leukotriene E4-induced airway hyperresponsiveness of guinea pig tracheal smooth muscle to histamine and evidence for three separate sulfidopeptide leukotriene receptors. Proc Natl Acad Sci USA, 81 (15): 4922-5. [PMID:6087352]
30. Lynch KR, O'Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z et al.. (1999) Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature, 399 (6738): 789-93. [PMID:10391245]
31. Maekawa A, Balestrieri B, Austen KF, Kanaoka Y. (2009) GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc Natl Acad Sci USA, 106 (28): 11685-90. [PMID:19561298]
32. Maekawa A, Kanaoka Y, Xing W, Austen KF. (2008) Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci USA, 105 (43): 16695-700. [PMID:18931305]
33. Muro S, Hamid Q, Olivenstein R, Taha R, Rokach J, Powell WS. (2003) 5-oxo-6,8,11,14-eicosatetraenoic acid induces the infiltration of granulocytes into human skin. J Allergy Clin Immunol, 112 (4): 768-74. [PMID:14564360]
34. Nagy E, Andersson DC, Caidahl K, Eriksson MJ, Eriksson P, Franco-Cereceda A, Hansson GK, Bäck M. (2011) Upregulation of the 5-lipoxygenase pathway in human aortic valves correlates with severity of stenosis and leads to leukotriene-induced effects on valvular myofibroblasts. Circulation, 123 (12): 1316-25. [PMID:21403093]
35. Ni NC, Yan D, Ballantyne LL, Barajas-Espinosa A, St Amand T, Pratt DA, Funk CD. (2011) A selective cysteinyl leukotriene receptor 2 antagonist blocks myocardial ischemia/reperfusion injury and vascular permeability in mice. J Pharmacol Exp Ther, 339 (3): 768-78. [PMID:21903747]
36. Nielsen CK, Campbell JI, Ohd JF, Mörgelin M, Riesbeck K, Landberg G, Sjölander A. (2005) A novel localization of the G-protein-coupled CysLT1 receptor in the nucleus of colorectal adenocarcinoma cells. Cancer Res, 65 (3): 732-42. [PMID:15705869]
37. Nonaka Y, Hiramoto T, Fujita N. (2005) Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun, 337: 281-288. [PMID:16185654]
38. O'Flaherty JT, Rogers LC, Paumi CM, Hantgan RR, Thomas LR, Clay CE, High K, Chen YQ, Willingham MC, Smitherman PK et al.. (2005) 5-Oxo-ETE analogs and the proliferation of cancer cells. Biochim Biophys Acta, 1736 (3): 228-36. [PMID:16154383]
39. Okuno T, Iizuka Y, Okazaki H, Yokomizo T, Taguchi R, Shimizu T. (2008) 12(S)-Hydroxyheptadeca-5Z, 8E, 10E-trienoic acid is a natural ligand for leukotriene B4 receptor 2. J Exp Med, 205 (4): 759-66. [PMID:18378794]
40. Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, Kanaoka Y, Conley P, Boyce JA. (2009) Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med, 206 (11): 2543-55. [PMID:19822647]
41. Powell WS, Chung D, Gravel S. (1995) 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of human eosinophil migration. J Immunol, 154 (8): 4123-32. [PMID:7706749]
42. Powell WS, Gravel S, Gravelle F. (1995) Formation of a 5-oxo metabolite of 5,8,11,14,17-eicosapentaenoic acid and its effects on human neutrophils and eosinophils. J Lipid Res, 36 (12): 2590-8. [PMID:8847485]
43. Powell WS, Gravel S, MacLeod RJ, Mills E, Hashefi M. (1993) Stimulation of human neutrophils by 5-oxo-6,8,11,14-eicosatetraenoic acid by a mechanism independent of the leukotriene B4 receptor. J Biol Chem, 268 (13): 9280-6. [PMID:8387490]
44. Powell WS, Gravelle F, Gravel S. (1992) Metabolism of 5(S)-hydroxy-6,8,11,14-eicosatetraenoic acid and other 5(S)-hydroxyeicosanoids by a specific dehydrogenase in human polymorphonuclear leukocytes. J Biol Chem, 267 (27): 19233-41. [PMID:1326548]
45. Price D, Musgrave SD, Shepstone L, Hillyer EV, Sims EJ, Gilbert RF, Juniper EF, Ayres JG, Kemp L, Blyth A et al.. (2011) Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med, 364 (18): 1695-707. [PMID:21542741]
46. Ravasi S, Capra V, Mezzetti M, Nicosia S, Rovati GE. (2000) A kinetic binding study to evaluate the pharmacological profile of a specific leukotriene C(4) binding site not coupled to contraction in human lung parenchyma. Mol Pharmacol, 57 (6): 1182-9. [PMID:10825389]
47. Ravasi S, Capra V, Panigalli T, Rovati GE, Nicosia S. (2002) Pharmacological differences among CysLT(1) receptor antagonists with respect to LTC(4) and LTD(4) in human lung parenchyma. Biochem Pharmacol, 63 (8): 1537-46. [PMID:11996896]
48. Sakata K, Bäck M. (2002) Receptor preferences of cysteinyl-leukotrienes in the guinea pig lung parenchyma. Eur J Pharmacol, 436 (1-2): 119-26. [PMID:11834255]
49. Serhan CN. (2007) Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol, 25: 101-37. [PMID:17090225]
50. Serhan CN, Petasis NA. (2011) Resolvins and protectins in inflammation resolution. Chem Rev, 111 (10): 5922-43. [PMID:21766791]
51. Shao WH, Del Prete A, Bock CB, Haribabu B. (2006) Targeted disruption of leukotriene B4 receptors BLT1 and BLT2: a critical role for BLT1 in collagen-induced arthritis in mice. J Immunol, 176 (10): 6254-61. [PMID:16670336]
52. Snyder DW, Krell RD. (1984) Pharmacological evidence for a distinct leukotriene C4 receptor in guinea-pig trachea. J Pharmacol Exp Ther, 231 (3): 616-22. [PMID:6094796]
53. Stanke-Labesque F, Molière P, Bessard J, Laville M, Véricel E, Lagarde M. (2008) Effect of dietary supplementation with increasing doses of docosahexaenoic acid on neutrophil lipid composition and leukotriene production in human healthy volunteers. Br J Nutr, 100 (4): 829-33. [PMID:18304388]
54. Sundaram S, Ghosh J. (2006) Expression of 5-oxoETE receptor in prostate cancer cells: critical role in survival. Biochem Biophys Res Commun, 339 (1): 93-8. [PMID:16289380]
55. Terawaki K, Yokomizo T, Nagase T, Toda A, Taniguchi M, Hashizume K, Yagi T, Shimizu T. (2005) Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J Immunol, 175 (7): 4217-25. [PMID:16177061]
56. Walch L, Norel X, Bäck M, Gascard JP, Dahlén SE, Brink C. (2002) Pharmacological evidence for a novel cysteinyl-leukotriene receptor subtype in human pulmonary artery smooth muscle. Br J Pharmacol, 137 (8): 1339-45. [PMID:12466244]
57. Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. (2009) International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev, 61 (2): 119-61. [PMID:19498085]
58. Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. (1997) A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature, 387 (6633): 620-4. [PMID:9177352]
59. Yokomizo T, Kato K, Hagiya H, Izumi T, Shimizu T. (2001) Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J Biol Chem, 276 (15): 12454-9. [PMID:11278893]
60. Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. (2000) A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med, 192 (3): 421-32. [PMID:10934230]
61. Yokota Y, Inoue H, Matsumura Y, Nabeta H, Narusawa M, Watanabe A, Sakamoto C, Hijikata Y, Iga-Murahashi M, Takayama K et al.. (2012) Absence of LTB4/BLT1 axis facilitates generation of mouse GM-CSF-induced long-lasting antitumor immunologic memory by enhancing innate and adaptive immune systems. Blood, 120 (17): 3444-54. [PMID:22936657]







