
GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Urotensin receptor: Introduction
General
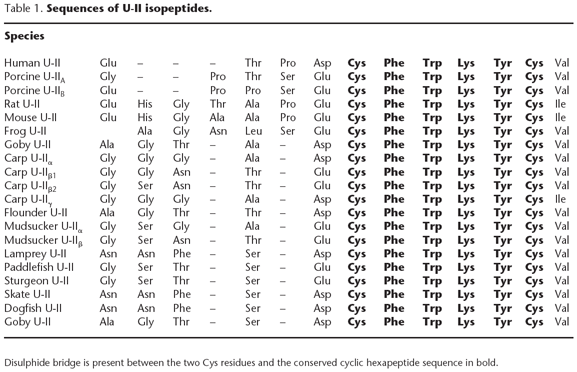
Urotensin-II (U-II), a cyclic dodecapeptide, was originally isolated [2] and purified [29] from urophysial extracts of the gobiid teleost Gillichthys mirabilis (long-jawed mudsucker). Initially, U-II isopeptides were shown to be distributed widely across evolutionary levels ranging from invertebrate gastropods (molluscs) to fish [3-5]. More recently, however, a human U-II isoform (11 residue peptide) has been identified [1,7], the direct result of the molecular cloning of fish [28] and amphibian [1] U-II cDNAs. Subsequently, additional mammalian isoforms have been identified in rodents (14 residues in mouse and rat) [6] and pig (12 residues) [25] as shown in Table 1.
As with many other polypeptide neurohormones (e.g. enkephalins, endothelins, somatostatin, cortistatin, orexins etc.), U-II isopeptides are derived from larger prepropeptide precursors. In man, two U-II propeptides have been identified (124 and 139 residue polypeptides [1,7]) believed to be alternate splice variants derived from a common gene (splice variants have been reported in the pig [22]). Both human precursor polypeptides encode an identical 11 amino acid residue mature peptide liberated via a putative polybasic (KKR) cleavage site. As seen in Table 1, distinct mature isopeptides have been identified in the carp, sucker and pig. For example, two homologous, but distinct, carp preproU-II cDNAs exist but, since they share greater than 90% identity, they are most likely derived from a common ancestral gene that has undergone one or more duplication events [28]. Although two isoforms of mature porcine U-II have been identified in spinal cord extracts (both dodecapeptides differing by a single amino acid in their N-terminus; Table 1), to date only one 121 residue propeptide has been cloned [22] (interestingly, it is estimated that ~50% of porcine preproU-II cDNAs also contain a 'silent' C>T at Asp [8]). Although the N-termini of preproU-IIs are extremely divergent (e.g. porcine preproU-II shares over 60% identity with human preproU-II but less than 10% with carp) [22], all cloned cDNAs contain amino-signal sequences (indicative of secreted proteins) and multiple internal mono/polybasic processing sites. It is currently unknown if additional bioactive proteolytic fragments are encoded by the preproU-II gene or whether the precursor peptide is recognized by specific 'convertases'.
In rat and human, Northern blot analysis and in situ hybridization reveals abundant expression of preproU-II expression in the brainstem (medulla oblongata) and spinal cord (specifically acetylcholinesterase-positive ventral horn motoneurones, confirmed immunohistochemically) [1,6-7]. In addition, U-II-like immunoreactivity is also present outside of the CNS, in particular within the vasculature [1] (low level mRNA expression has been reported in kidney, pituitary etc.). Indeed, consistent with a circulating endocrine/neurohumoral action, U-II-like immunoreactivity is present in plasma of fish [20]. However, it remains to be seen if U-II circulates in a free, bioactive form in man or whether it is secreted into the blood in the form of an inactive precursor/associated with a carrier protein(s).
Receptor identification
The presence of a FWK tripeptide motif within the cyclized region of all U-II isoforms originally led to this peptide family being described as 'somatostatin or cortistatin-like'. However, despite the apparent structural similarity, U-II and somatostatin are not homologous. Indeed, although it was originally proposed that U-II and somatostatin shared a common binding site [5], both binding ([125I]-radiolabelled goby urotensin-II in rat aortic membranes) and functional (aortic contraction) studies demonstrated that U-II mediated its biological action(s) via unique 'U-II-specific receptor(s)'. This was demonstrated unequivocally by Ames et al. [1] who, using 'reverse pharmacology' [31], identified U-II as the cognate ligand for a novel rat orphan G protein-coupled receptor (386 residue) originally designated as SENR (sensory epithelial neuropeptide-like receptor) [32] or GPR14 [24]. This receptor is hereby designated the UT receptor. Subsequently, this ligand pairing has been confirmed (using rat GPR14) by three additional groups using Ca2+-mobilization (aequorin bioluminescence/FLIPR) and [3H]-arachidonate metabolism assays in transient and stable CHO and HEK293 cell lines [22,25,27].
Phylogenetically, this intronless receptor family is most closely related to the rhodopsin G protein-coupled receptor family, showing ~25% identity overall with somatostatin (SST2 and SST4), opioid (κ, δ and μ) and galanin receptors. The level of identity increases to ~40% within the hydrophobic transmembrane domains (TM). Indeed, rat GPR14 was originally PCR-cloned using oligonucleotide primers designed against conserved nucleotide sequences within the TMs of somatostatin or opioid receptor family. It is also of interest to note that the Asp residue present in TM3 of somatostatin and opioid receptors (essential for binding), is also present in GPR14 (the significance of which awaits elucidation). GPR14 also shares homology to two additional orphan receptors, GPR7 and GPR8 but U-II is a ligand for neither.
Once U-II was identified as the cognate ligand for rat GPR14, Ames et al. [1] cloned a 389 residue U-II receptor homologue in man (75% identity with rat GPR14/SENR, the majority of sequence differences occurring at the N- and C-termini and in the third intracellular loop). Receptors from both species exhibit the characteristic lipophilic 7TM structure of the G protein-coupled superfamily and both possess two potential N-linked glycosylation sites in the N-terminus. Conserved Cys123/Cys199 residues are present in the second and third extracellular loops (putative disulphide bridge) and putative intracellular PKA/PKC phosphorylation sites are evident in the second and third intracellular loops. Although an NPXXY consensus motif is retained in TM7 of both species (required for receptor internalization), the putative palmitoylation site (Cys399) present in rat is absent in the human receptor [1,24,32].
Preliminary immunohistochemical and biochemical studies demonstrated the presence of U-II-like activity in brain extracts from fish and amphibian. Based on the expression of preproU-II cDNA within the mammalian CNS (see above), it is of interest to note that, recently, rp-HPLC fractions have been identified in porcine spinal cord [25], bovine hypothalamic and squirrel monkey brain [27] extracts that selectively activate the recombinant urotensin-II receptor. Since activity was sensitive to trypsin and alkylating agents, it was concluded that this activity was the result of a peptidergic constituent.
Pharmacological characteristics
The UT receptor, transiently and stably expressed in recombinant HEK293, CHO and COS-7 host cells expressing rat or human receptor [1,22,25,27], is selective for U-II since from over 700 ligands studied (polypeptides, lipids, biogenic amines etc.), only the U-II isopeptides behaved as UT agonists [1]. This activity was not, for example, shared by high concentrations (<1μM) of SRIF-14, cortistatin-14, urotensin-I, [Arg8]vasopressin, angiotensin II, MCH, sCT or NPY [1,22,25,27].
Radioligand binding assays [1,22,27] revealed Kd 0.07-0.40nM in recombinant cells. Although Bmax is reported to be high in stable cell lines [1,27], to date, significantly lower 'endogenous' receptor densities have been reported in native mammalian tissues [1,5,18,21]. This receptor does not distinguish between fish, frog and human isopeptides [1,22,25,27] where nanomolar affinities are found in both recombinant cell- and tissue-based binding and functional assays.
The implication that the divergent N-terminus of the U-II isopeptides does not contribute to ligand binding or agonist activity is entirely consistent with the observation that full affinity and agonist biological activity (potency and efficacy) is retained by the highly conserved core cyclic octapeptide sequence of the U-II isopeptide family [5,17-18].
Although it has been postulated that receptor-mediated Ca2+ elevations are transduced by Gαq/11 in HEK 293 cells [22], this phenomenon has not been studied in detail, either in transfected cells or native cells expressing urotensin receptor(s). As such, the specific G protein(s) that couple with this receptor family awaits elucidation.
Northern blot, dot blot, RT-PCR and in situ hybridization reveal that rat, mouse and human UT is expressed in a variety of tissues (see data tables). RNase protection (rat probe) also reveals expression in bovine cerebellum and heart. Although it has been proposed that receptor splice variants exist (e.g. UII-R1a) [22], the experimental data, as such, are insufficient to support such a hypothesis.
Peptide analogue structure-activity relations
Although the N-terminus of U-II is divergent across species (Table 1), all U-II isoforms share a conserved cyclic hexapeptide core sequence motif (CFWKYC) immediately flanked by acidic (amino -D/E) and neutral (carboxy-V/I) amino acids. Indeed, the resultant octapeptide retains full biological activity. The contractile potencies (EC50) of goby urotensin-II and its truncated analogues, goby [5-12]U-II, goby [6-12]U-II and goby [5-11]U-II, are 0.7, 0.5, 3.0 and >100 nM, respectively in rat isolated aortae [4,17-18]. Similarly, this rank order of contractile potency was in agreement with radioligand binding studies in rat aortic membranes. Consistent with this, the pharmacology of goby, frog, porcine and human U-II is indistinguishable both in binding and functional studies [1,22]. In addition, a [D-Trp7]-substituted human U-II analogue (acetyl-[5-10]human U-II-amide) is ~200-fold less potent than the parent peptide at rat UT receptor [27]. Relative to human and goby U-II (EC50 = 0.10 and 0.14nM, respectively), somatostatin-14 (EC50 = 3.7μM) and its analogues RC160 (338nM) and ocreotide (4.3μM) were three or four orders of magnitude less potent as functional agonists at this receptor [27].
Biological responses mediated by urotensin-II isopeptides
Since evolutionary pressure has acted to conserve the biologically active sequence of U-II, even prior to its cloning, the implication was that U-II exerted important physiological actions in man. Indeed, although the fish urophysis represents a vestigal neurohaemal organ present at the caudal portion of the spinal cord, it is functionally and morphologically analogous to the mammalian hypothalamo-neurohypophysial axis. Nevertheless, to date, the physiological actions of the U-II isopeptides remain poorly defined (for reviews see [3-5]) and, with the exception perhaps of the vascular effects, are primarily derived from functional studies performed in non-mammalian species (in particular, concentration-response relations are ill determined).
Vascular activity
U-II isopeptides are clearly potent (EC50 = 0.1-2.4nM), sustained mammalian vasoconstrictors in vitro (although anatomical and species difference are evident). In addition to non-mammalian isolated vascular tissue [4], U-II isopeptides, either synthetic or purified fish urophysis extract, alter vascular tone in a variety of mammalian blood vessels including those isolated from rats [1,9,11,13,16-18,27], rabbits [26], pigs, dogs [9] and non-human primates [1,9]. This phenomenon is observed primarily in arterial vasculature, consistent with the expression of UT in cardiac and arterial, but not venous, blood vessels and smooth muscle cell lines [1].
Vasoconstriction is resistant to block of adrenergic or cholinergic systems (phentolamine, propranolol, atropine), 5-HT or histamine receptor antagonism, Na+ channel block (TTX, cocaine) but is dependent on extracellular Ca2+ influx (i.e. EGTA- and nitrendipine/verapamil-sensitive) and inhibited by calmodulin antagonists [11,13,18]. Since responses are reported to be phospholipase-sensitive, such an effect is likely secondary to phosphoinositide metabolism [13]. This would be consistent with observations made using recombinant receptors where receptor activation is coupled to [Ca2+]i and [3H]-arachidonate hydrolysis in HEK 293, CHO and COS-7 cells [1,22,25,27].
Although endothelium-dependent relaxation has been described, destruction of the endothelium or inhibition of cyclo- and lipo-oxygenase and nitric oxide synthase does not radically alter the contractile actions of U-II in the isolated thoracic aorta of the rat [26] (although both L-NAME and indomethacin partially attenuate the in vivo systemic depressor actions of goby U-II in the rat [5,15,33]). Although U-II induces complex haemodynamic effects in vivo (both pressor and depressor actions have been reported) [3,5,14-15,33], the peptide clearly increases in total peripheral resistance (accompanied by profound cardiac depressor actions) in anaesthetized primates [1].
Non-vascular smooth muscle function
U-II isopeptides influence 'non-vascular' smooth muscle function; U-II alters piscine and amphibian gastrointestinal (ileum, rectum) and genitourinary (bladder, oviduct and sperm duct) contractile function [2-5,12]. Effects in mammalian smooth muscle activity have been recorded e.g. altered tone in the mouse anococcygeus muscle [23]. Although such a postulate awaits confirmation, the possibility exists that U-II influences neuromuscular physiology since the ligand is present in ventral horn motoneurones and the receptor expression is present in skeletal muscle.
Osmoregulation
U-II modulates transepithelial Na+/Cl- ion transport in piscine skin epithelia, operculum, intestine and bladder [3-5,19] indicating that, at least in fish, this isopeptide family possesses an osmoregulatory function. However, similar effects in mammals await elucidation (although renal preproU-II mRNA expression has been reported [6], it is unknown if this peptide regulates renal function).
Metabolic actions
U-II may regulate endocrine and metabolic function. Elevations in circulating catecholamines and cortisol have been observed in dogfish and flounder (the latter steroidogenic action synergises with ACTH and, interestingly, is sensitive to stress-induced or osmoregulatory challenges) [3-5,30]. U-II also enhances lipid mobilization and inhibits prolactin release in fish [3-5,10].
Issues
Although receptor expression is observed within the arterial vasculature, the lack of selective antagonists or knock-out or transgenic animals precludes the demonstration that U-II-mediated functional responses are transduced by the cloned (GPR14/SENR) receptor. Indeed, although additional receptor types may exist, no functional or molecular data exist, as of yet, to support such a hypothesis (although receptor splice variants have been proposed [27]). Similarly, it is unclear whether the rat and human receptor are orthologues or paralogues (they share <80% identity). Similarly, it is not known currently which signalling pathways are involved in transducing responses to U-II, either those mediated in recombinant receptor systems or by 'endogenous' receptors [1,3-5,9,13-14,16-18,21-22,25-26]. With the exception of the vascular effects, most functional gastrointestinal/genitourinary, osmoregulatory and metabolic effects have been reported in non-mammalian tissue. Indeed, to date, the detailed concentration-response relationships are poorly defined in many systems. This will, hopefully, be greatly aided in the future by the generation of divergent U-II analogues, receptor antagonists, knockout or transgenic animals and detailed cloning approaches.
References
1. Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, Louden CS, Foley JJ, Sauermelch CF, Coatney RW et al.. (1999) Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature, 401 (6750): 282-6. [PMID:10499587]
2. Bern HA, Lederis K. (1969) A reference preparation for the study of active substances in the caudal neurosecretory system of teleosts. J Endocrinol, 45 (1): Suppl:xi-xii. [PMID:5347394]
3. Bern HA, Pearson D, Larson BA, Nishioka RS. (1985) Neurohormones from fish tails: the caudal neurosecretory system. I: "Urophysiology" and the caudal neurosecretory system of fishes. Recent Prog Horm Res, 41: 533-552. [PMID:2864726]
4. Conlon JM, Tostivint H, Vaudry H. (1997) Somatostatin- and urotensin II-related peptides: molecular diversity and evolutionary perspectives. Regul Pept, 69 (2): 95-103. [PMID:9178352]
5. Conlon JM, Yano K, Waugh D, Hazon N. (1996) Distribution and molecular forms of urotensin II and its role in cardiovascular regulation in vertebrates. J Exp Zool, 275: 226-238. [PMID:8676097]
6. Coulouarn Y, Jégou S, Tostivint H, Vaudry H, Lihrmann I. (1999) Cloning, sequence analysis and tissue distribution of the mouse and rat urotensin II precursors. FEBS Lett, 457 (1): 28-32. [PMID:10486557]
7. Coulouarn Y, Lihrmann I, Jegou S, Anouar Y, Tostivint H, Beauvillain JC, Conlon JM, Bern HA, Vaudry H. (1998) Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc Natl Acad Sci USA, 95 (26): 15803-8. [PMID:9861051]
8. Coy DH, Rossowski WJ, Cheng BL, Taylor JE. (2002) Structural requirements at the N-terminus of urotensin II octapeptides. Peptides, 23 (12): 2259-64. [PMID:12535707]
9. Douglas SA, Sulpizio AC, Piercy V, Sarau HM, Ames RS, Aiyar NV, Ohlstein EH, Willette RN. (2000) Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br J Pharmacol, 131 (7): 1262-74. [PMID:11090097]
10. Elshourbagy NA, Douglas SA, Shabon U, Harrison S, Duddy G, Sechler JL, Ao Z, Maleeff BE, Naselsky D, Disa J et al.. (2002) Molecular and pharmacological characterization of genes encoding urotensin-II peptides and their cognate G-protein-coupled receptors from the mouse and monkey. Br J Pharmacol, 136 (1): 9-22. [PMID:11976263]
11. Gibson A. (1987) Complex effects of Gillichthys urotensin II on rat aortic strips. Br J Pharmacol, 91 (1): 205-12. [PMID:2885055]
12. Gibson A, Bern HA, Ginsburg M, Botting JH. (1984) Neuropeptide-induced contraction and relaxation of the mouse anococcygeus muscle. Proc Natl Acad Sci USA, 81 (2): 625-9. [PMID:6582516]
13. Gibson A, Conyers S, Bern HA. (1988) The influence of urotensin II on calcium flux in rat aorta. J Pharm Pharmacol, 40 (12): 893-5. [PMID:2907588]
14. Gibson A, Wallace P, Bern HA. (1986) Cardiovascular effects of urotensin II in anesthetized and pithed rats. Gen Comp Endocrinol, 64 (3): 435-9. [PMID:2879765]
15. Hasegawa K, Kobayashi Y, Kobayashi H. (1992) Vasodepressor effects of urotensin II in rats. Neuro Endocrinol Lett, 14: 357-363.
16. Itoh H, Higuchi H, Hiraoka N, Ito M, Konishi T, Nakano T, Lederis K. (1991) Contraction of rat thoracic aorta strips by endothelin-1 in the absence of extracellular Ca2+. Br J Pharmacol, 104 (4): 847-52. [PMID:1810598]
17. Itoh H, Itoh Y, Rivier J, Lederis K. (1987) Contraction of major artery segments of rat by fish neuropeptide urotensin II. Am J Physiol, 252 (2 Pt 2): R361-6. [PMID:3812773]
18. Itoh H, McMaster D, Lederis K. (1988) Functional receptors for fish neuropeptide urotensin II in major rat arteries. Eur J Pharmacol, 149 (1-2): 61-6. [PMID:3396626]
19. Kelsall CJ, Balment RJ. (1998) Native urotensins influence cortisol secretion and plasma cortisol concentration in the euryhaline flounder, platichthys flesus. Gen Comp Endocrinol, 112 (2): 210-9. [PMID:9784304]
20. Kobayashi Y, Lederis K, Rivier J, Ko D, McMaster D, Poulin P. (1986) Radioimmunoassays for fish tail neuropeptides: II. Development of a specific and sensitive assay for and the occurrence of immunoreactive urotensin II in the central nervous system and blood of Catostomus commersoni. J Pharmacol Methods, 15 (4): 321-33. [PMID:3724202]
21. Lederis K. (1984) The fish urotensins: hypophyseal and peripheral actions in fishes and mammals. In Frontiers in neuroendocrinology Edited by Martin L, Ganong WF (Raven Press) 247-263.
22. Liu Q, Pong SS, Zeng Z, Zhang Q, Howard AD, Williams Jr DL, Davidoff M, Wang R, Austin CP, McDonald TP et al.. (1999) Identification of urotensin II as the endogenous ligand for the orphan G-protein-coupled receptor GPR14. Biochem Biophys Res Commun, 266 (1): 174-8. [PMID:10581185]
23. Loretz CA, Howard ME, Siegel AJ. (1985) Ion transport in goby intestine: cellular mechanism of urotensin II stimulation. Am J Physiol, 249 (2 Pt 1): G284-93. [PMID:2411149]
24. Marchese A, Heiber M, Nguyen T, Heng HH, Saldivia VR, Cheng R, Murphy PM, Tsui LC, Shi X, Gregor P et al.. (1995) Cloning and chromosomal mapping of three novel genes, GPR9, GPR10, and GPR14, encoding receptors related to interleukin 8, neuropeptide Y, and somatostatin receptors. Genomics, 29 (2): 335-44. [PMID:8666380]
25. Mori M, Sugo T, Abe M, Shimomura Y, Kurihara M, Kitada C, Kikuchi K, Shintani Y, Kurokawa T, Onda H et al.. (1999) Urotensin II is the endogenous ligand of a G-protein-coupled orphan receptor, SENR (GPR14). Biochem Biophys Res Commun, 265 (1): 123-9. [PMID:10548501]
26. Muramatsu I, Fujiwara M, Hidaka H, Akutagawa H. (1979) Pharmacological analysis of urotensin-induced contraction and relaxation in isolated rabbit aortas. Gunma Symp Endocrinol, 16: 39-47.
27. Nothacker HP, Wang Z, McNeill AM, Saito Y, Merten S, O'Dowd B, Duckles SP, Civelli O. (1999) Identification of the natural ligand of an orphan G-protein-coupled receptor involved in the regulation of vasoconstriction. Nat Cell Biol, 1 (6): 383-5. [PMID:10559967]
28. Ohsako S, Ishida I, Ichikawa T, Deguchi T. (1986) Cloning and sequence analysis of cDNAs encoding precursors of urotensin II-alpha and -gamma. J Neurosci, 6 (9): 2730-5. [PMID:2427672]
29. Pearson D, Shively JE, Clark BR, Geschwind II, Barkley M, Nishioka RS, Bern HA. (1980) Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc Natl Acad Sci USA, 77 (8): 5021-4. [PMID:6107911]
30. Sheridan MA, Bern HA. (1986) Both somatostatin and the caudal neuropeptide, urotensin II, stimulate lipid mobilization from coho salmon liver incubated in vitro. Regul Pept, 14 (4): 333-44. [PMID:2428079]
31. Stadel JM, Wilson S, Bergsma DJ. (1997) Orphan G protein-coupled receptors: a neglected opportunity for pioneer drug discovery. Trends Pharmacol Sci, 18 (11): 430-7. [PMID:9426471]
32. Tal M, Ammar DA, Karpuj M, Krizhanovsky V, Naim M, Thompson DA. (1995) A novel putative neuropeptide receptor expressed in neural tissue, including sensory epithelia. Biochem Biophys Res Commun, 209 (2): 752-9. [PMID:7733947]
33. Yano K, Vaudry H, Conlon JM. (1994) Spasmogenic actions of frog urotensin II on the bladder and ileum of the frog, Rana catesbeiana. Gen Comp Endocrinol, 96 (3): 412-9. [PMID:7883148]







