
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Target not currently curated in GtoImmuPdb
Target id: 1785
Nomenclature: activin A receptor type 1
Abbreviated Name: ALK2
| Quaternary Structure: Complexes |
| Bone morphogenetic protein receptors |
| Anti-Müllerian hormone receptors |
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 1 | 509 | 2q24.1 | ACVR1 | activin A receptor type 1 | |
| Mouse | 1 | 509 | 2 C1.1 | Acvr1 | activin A receptor, type 1 | |
| Rat | 1 | 509 | 3q21 | Acvr1 | activin A receptor type 1 | |
Database Links  |
|
| Alphafold | Q04771 (Hs), P37172 (Mm), P80201 (Rn) |
| BRENDA | 2.7.11.30 |
| ChEMBL Target | CHEMBL5903 (Hs), CHEMBL3309042 (Mm) |
| Ensembl Gene | ENSG00000115170 (Hs), ENSMUSG00000026836 (Mm), ENSRNOG00000005033 (Rn) |
| Entrez Gene | 90 (Hs), 11477 (Mm), 79558 (Rn) |
| Human Protein Atlas | ENSG00000115170 (Hs) |
| KEGG Enzyme | 2.7.11.30 |
| KEGG Gene | hsa:90 (Hs), mmu:11477 (Mm), rno:79558 (Rn) |
| OMIM | 102576 (Hs) |
| Orphanet | ORPHA117759 (Hs) |
| Pharos | Q04771 (Hs) |
| RefSeq Nucleotide | NM_001105 (Hs), NM_001110205 (Mm), NM_024486 (Rn) |
| RefSeq Protein | NP_001096 (Hs), NP_001103675 (Mm), NP_001103674 (Mm), NP_031420 (Mm), NP_077812 (Rn) |
| UniProtKB | Q04771 (Hs), P37172 (Mm), P80201 (Rn) |
| Wikipedia | ACVR1 (Hs) |
Selected 3D Structures  |
|||||||||||||
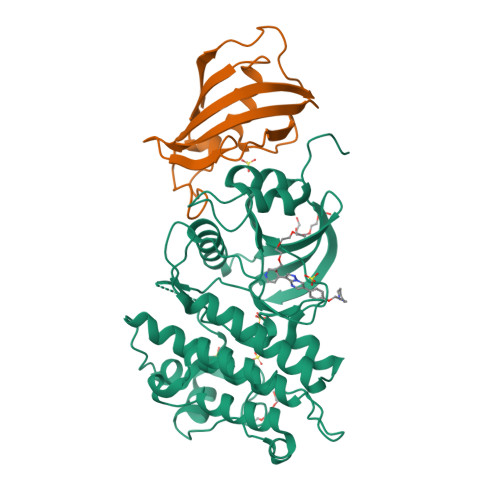
|
|
||||||||||||
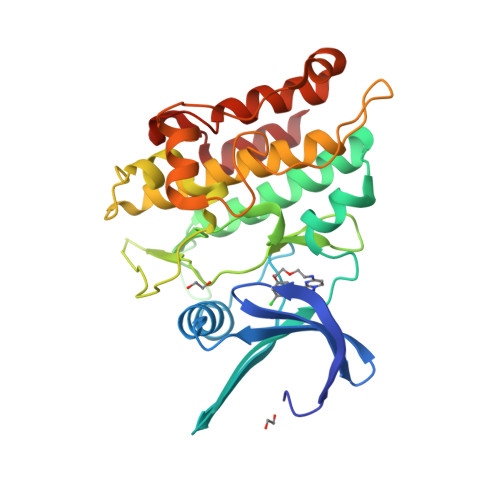
|
|
||||||||||||
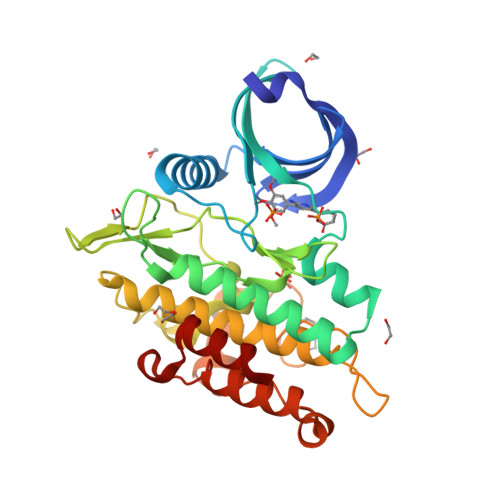
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 6,14 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: ACVR1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: ...1 |

|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: nd/ALK2(ACVR1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immuno Process Associations | ||||||||||||
|
||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||
|
||||||||||||
1. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377]
2. Arista L, Babu S, Bian J, Cui K, Dillon MP, Lattmann R, Li J, Liao L, Lizos D, Ramos R et al.. (2021) Aminopyridine derivatives and their use as selective alk-2 inhibitors. Patent number: US20210155606A1. Assignee: Novartis AG. Priority date: 20/07/2016. Publication date: 27/05/2021.
3. Asshoff M, Petzer V, Warr MR, Haschka D, Tymoszuk P, Demetz E, Seifert M, Posch W, Nairz M, Maciejewski P et al.. (2017) Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood, 129 (13): 1823-1830. [PMID:28188131]
4. Brooijmans N, Brubaker JD, Fleming PE, Hodous BL, Kim JL, Waetzig J, Williams B, Wilson D, Wilson KJ, Cronin M. (2017) Inhibitors of activin receptor-like kinase. Patent number: WO2017181117A1. Assignee: Blueprint Medicines. Priority date: 15/04/2016. Publication date: 19/10/2017.
5. Chaikuad A, Alfano I, Kerr G, Sanvitale CE, Boergermann JH, Triffitt JT, von Delft F, Knapp S, Knaus P, Bullock AN. (2012) Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem, 287 (44): 36990-8. [PMID:22977237]
6. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
7. Engers DW, Frist AY, Lindsley CW, Hong CC, Hopkins CR. (2013) Synthesis and structure-activity relationships of a novel and selective bone morphogenetic protein receptor (BMP) inhibitor derived from the pyrazolo[1.5-a]pyrimidine scaffold of dorsomorphin: the discovery of ML347 as an ALK2 versus ALK3 selective MLPCN probe. Bioorg Med Chem Lett, 23 (11): 3248-52. [PMID:23639540]
8. Mohedas AH, Wang Y, Sanvitale CE, Canning P, Choi S, Xing X, Bullock AN, Cuny GD, Yu PB. (2014) Structure-activity relationship of 3,5-diaryl-2-aminopyridine ALK2 inhibitors reveals unaltered binding affinity for fibrodysplasia ossificans progressiva causing mutants. J Med Chem, 57 (19): 7900-15. [PMID:25101911]
9. Mollard A, Warner SL, Flymm GA, Vankayalapati H, Bearss DJ. (2014) Jak2 and alk2 inhibitors and methods for their use. Patent number: WO2014151871A9. Assignee: Tolero Pharmaceuticals, Inc.. Priority date: 14/03/2013. Publication date: 31/12/2014.
10. Nguyen MH, Ye HF, Xu Y, Truong L, Horsey A, Styduhar ED, Frascella M, Leffet L, Federowicz K, Behshad E et al.. (2023) Discovery of Orally Bioavailable FGFR2/FGFR3 Dual Inhibitors via Structure-Guided Scaffold Repurposing Approach. ACS Medicinal Chemistry Letters, 14 (3): 312–318. DOI: 10.1021/acsmedchemlett.3c00003
11. Sanvitale CE, Kerr G, Chaikuad A, Ramel MC, Mohedas AH, Reichert S, Wang Y, Triffitt JT, Cuny GD, Yu PB et al.. (2013) A new class of small molecule inhibitor of BMP signaling. PLoS One, 8 (4): e62721. [PMID:23646137]
12. Velaparthi U, Darne CP, Warrier J, Liu P, Rahaman H, Augustine-Rauch K, Parrish K, Yang Z, Swanson J, Brown J et al.. (2020) Discovery of BMS-986260, a Potent, Selective, and Orally Bioavailable TGFβR1 Inhibitor as an Immuno-oncology Agent. ACS Med Chem Lett, 11 (2): 172-178. DOI: 10.1021/acsmedchemlett.9b00552 [PMID:32071685]
13. Witten MR, Wu L, Lai CT, Kapilashrami K, Pusey M, Gallagher K, Chen Y, Yao W. (2022) Inhibition of ALK2 with bicyclic pyridyllactams. Bioorg Med Chem Lett, 55: 128452. [PMID:34780900]
14. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
Type I receptor serine/threonine kinases: activin A receptor type 1. Last modified on 19/03/2024. Accessed on 09/07/2025. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1785.