
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 326 | 1q32.1 | ADORA1 | adenosine A1 receptor | 88,120,144 |
| Mouse | 7 | 326 | 1 E4 | Adora1 | adenosine A1 receptor | 97,155 |
| Rat | 7 | 326 | 13q13 | Adora1 | adenosine A1 receptor | 96 |
Previous and Unofficial Names  |
| RDC7 | adenosine receptor A1 | A1-AR | A1R |
Database Links  |
|
| Specialist databases | |
| GPCRdb | aa1r_human (Hs), aa1r_mouse (Mm), aa1r_rat (Rn) |
| Other databases | |
| Alphafold | P30542 (Hs), Q60612 (Mm), P25099 (Rn) |
| ChEMBL Target | CHEMBL226 (Hs), CHEMBL3688 (Mm), CHEMBL318 (Rn) |
| DrugBank Target | P30542 (Hs) |
| Ensembl Gene | ENSG00000163485 (Hs), ENSMUSG00000042429 (Mm), ENSRNOG00000003442 (Rn) |
| Entrez Gene | 134 (Hs), 11539 (Mm), 29290 (Rn) |
| Human Protein Atlas | ENSG00000163485 (Hs) |
| KEGG Gene | hsa:134 (Hs), mmu:11539 (Mm), rno:29290 (Rn) |
| OMIM | 102775 (Hs) |
| Pharos | P30542 (Hs) |
| RefSeq Nucleotide | NM_000674 (Hs), NM_001008533 (Mm), NM_017155 (Rn) |
| RefSeq Protein | NP_000665 (Hs), NP_001008533 (Mm), NP_058851 (Rn) |
| UniProtKB | P30542 (Hs), Q60612 (Mm), P25099 (Rn) |
| Wikipedia | ADORA1 (Hs) |
Selected 3D Structures  |
|||||||||||||
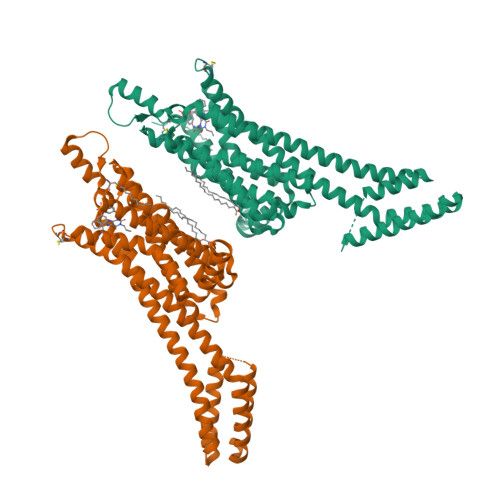
|
|
||||||||||||
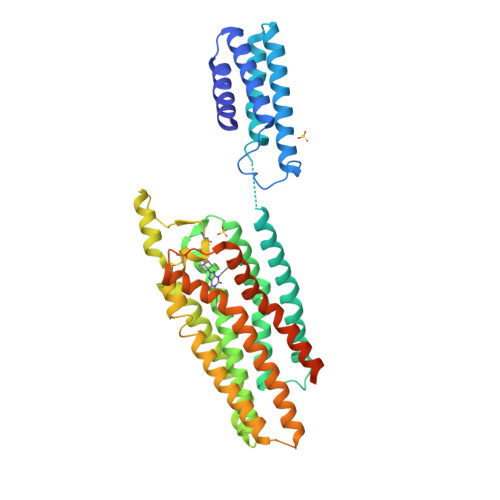
|
|
||||||||||||
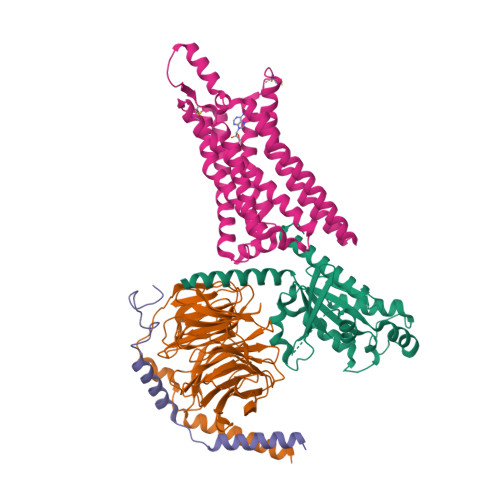
|
|
||||||||||||
Natural/Endogenous Ligands  |
| adenosine |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific agonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The role for adenosine as endogenous ligand for the A1 receptor is described in [39] and [40]. The role for adenosine as endogenous ligand for the A1 receptor is described in [40] and [41]. Capadenoson and neladenoson have been evaluated in phase 2 clinical trials for heart failure, but failed to meet expectations [152]. Primary target mapping: we have tagged the adenosine A1 receptor as the primary target for this endogenous ligand as the affinity is marginally higher at this receptor isoform. However, adenosine is likely to exert clinical effects via other adenosine receptor family members. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reference [28] does not use a transfected cell system but measures binding to the human hippocampus. Istradefylline pKi values are derived from unpublished data (Müller et al.). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulators | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulator Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| In vitro, VPC171 enhances A1R agonist ((R)-PIA)-induced ERK1/2 phosphorylation with a 2.4-fold positive cooperativity, and enhances [35S]GTPγS binding with a 6.9-fold positive cooperativity under the same conditions [61]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| Adenosine exerts anti-inflammatory effects on a number of immune cells types. These effects are mediated by the adenosine G portein-coupled receptors. All four adenosine receptors are expressed on the surface of mouse invariant NKT (iNKT) cells. The specific role of the A1 receptor in adenosine-mediated anti-inflammatory effects is not fully understood. |
| Cell Type Associations | ||||||||
|
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
| Gi/Go family | Adenylyl cyclase inhibition |
| References: 21,66 | |
Secondary Transduction Mechanisms  |
|
| Transducer | Effector/Response |
|
Gs family Gq/G11 family |
Adenylyl cyclase stimulation Phospholipase C stimulation |
| References: 21 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Comments |
| For a review of the effects of adenosine receptor knockout on nervous system function see reference [40]. |
1. Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A, Müller CE. (2004) Antinociceptive effects of novel A2B adenosine receptor antagonists. J Pharmacol Exp Ther, 308 (1): 358-66. [PMID:14563788]
2. Albrecht-Küpper BE, Leineweber K, Nell PG. (2012) Partial adenosine A1 receptor agonists for cardiovascular therapies. Purinergic Signal, 8 (Suppl 1): 91-9. [PMID:22081230]
3. Allegrucci C, Liguori L, Minelli A. (2001) Stimulation by n6-cyclopentyladenosine of A1 adenosine receptors, coupled to galphai2 protein subunit, has a capacitative effect on human spermatozoa. Biol Reprod, 64 (6): 1653-9. [PMID:11369591]
4. Anderson R, Sheehan MJ, Strong P. (1994) Characterization of the adenosine receptors mediating hypothermia in the conscious mouse. Br J Pharmacol, 113 (4): 1386-90. [PMID:7889296]
5. Arrigoni E, Crocker AJ, Saper CB, Greene RW, Scammell TE. (2005) Deletion of presynaptic adenosine A1 receptors impairs the recovery of synaptic transmission after hypoxia. Neuroscience, 132 (3): 575-80. [PMID:15837119]
6. Aurelio L, Valant C, Flynn BL, Sexton PM, Christopoulos A, Scammells PJ. (2009) Allosteric modulators of the adenosine A1 receptor: synthesis and pharmacological evaluation of 4-substituted 2-amino-3-benzoylthiophenes. J Med Chem, 52 (14): 4543-7. [PMID:19514747]
7. Baraldi PG, Tabrizi MA, Preti D, Bovero A, Romagnoli R, Fruttarolo F, Zaid NA, Moorman AR, Varani K, Gessi S et al.. (2004) Design, synthesis, and biological evaluation of new 8-heterocyclic xanthine derivatives as highly potent and selective human A2B adenosine receptor antagonists. J Med Chem, 47 (6): 1434-47. [PMID:14998332]
8. Betti M, Catarzi D, Varano F, Falsini M, Varani K, Vincenzi F, Pasquini S, di Cesare Mannelli L, Ghelardini C, Lucarini E et al.. (2019) Modifications on the Amino-3,5-dicyanopyridine Core To Obtain Multifaceted Adenosine Receptor Ligands with Antineuropathic Activity. J Med Chem, 62 (15): 6894-6912. [PMID:31306001]
9. Beukers MW, Wanner MJ, Von Frijtag Drabbe Künzel JK, Klaasse EC, IJzerman AP, Koomen GJ. (2003) N6-cyclopentyl-2-(3-phenylaminocarbonyltriazene-1-yl)adenosine (TCPA), a very selective agonist with high affinity for the human adenosine A1 receptor. J Med Chem, 46 (8): 1492-503. [PMID:12672250]
10. Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. (2009) Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci, 29 (5): 1267-76. [PMID:19193874]
11. Bonizzoni E, Milani S, Ongini E, Casati C, Monopoli A. (1995) Modeling hemodynamic profiles by telemetry in the rat. A study with A1 and A2a adenosine agonists. Hypertension, 25 (4 Pt 1): 564-9. [PMID:7721399]
12. Borodovsky A, Barbon CM, Wang Y, Ye M, Prickett L, Chandra D, Shaw J, Deng N, Sachsenmeier K, Clarke JD et al.. (2020) Small molecule AZD4635 inhibitor of A 2A R signaling rescues immune cell function including CD103 + dendritic cells enhancing anti-tumor immunity. J Immunother Cancer, 8 (2): e000417. DOI: 10.1136/jitc-2019-000417 [PMID:32727810]
13. Borrmann T, Hinz S, Bertarelli DC, Li W, Florin NC, Scheiff AB, Müller CE. (2009) 1-alkyl-8-(piperazine-1-sulfonyl)phenylxanthines: development and characterization of adenosine A2B receptor antagonists and a new radioligand with subnanomolar affinity and subtype specificity. J Med Chem, 52 (13): 3994-4006. [PMID:19569717]
14. Brown R, Ollerstam A, Johansson B, Skøtt O, Gebre-Medhin S, Fredholm B, Persson AE. (2001) Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol, 281 (5): R1362-7. [PMID:11641103]
15. Bruns RF, Fergus JH. (1990) Allosteric enhancement of adenosine A1 receptor binding and function by 2-amino-3-benzoylthiophenes. Mol Pharmacol, 38 (6): 939-49. [PMID:2174510]
16. Børglum JD, Vassaux G, Richelsen B, Gaillard D, Darimont C, Ailhaud G, Négrel R. (1996) Changes in adenosine A1- and A2-receptor expression during adipose cell differentiation. Mol Cell Endocrinol, 117 (1): 17-25. [PMID:8734470]
17. Cappellacci L, Franchetti P, Pasqualini M, Petrelli R, Vita P, Lavecchia A, Novellino E, Costa B, Martini C, Klotz KN et al.. (2005) Synthesis, biological evaluation, and molecular modeling of ribose-modified adenosine analogues as adenosine receptor agonists. J Med Chem, 48 (5): 1550-62. [PMID:15743197]
18. Chang LC, von Frijtag Drabbe Künzel JK, Mulder-Krieger T, Westerhout J, Spangenberg T, Brussee J, Ijzerman AP. (2007) 2,6,8-trisubstituted 1-deazapurines as adenosine receptor antagonists. J Med Chem, 50 (4): 828-34. [PMID:17300165]
19. Cheng RKY, Segala E, Robertson N, Deflorian F, Doré AS, Errey JC, Fiez-Vandal C, Marshall FH, Cooke RM. (2017) Structures of Human A1 and A2A Adenosine Receptors with Xanthines Reveal Determinants of Selectivity. Structure, 25 (8): 1275-1285.e4. [PMID:28712806]
20. Christofi FL, Zhang H, Yu JG, Guzman J, Xue J, Kim M, Wang YZ, Cooke HJ. (2001) Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system. J Comp Neurol, 439 (1): 46-64. [PMID:11579381]
21. Cordeaux Y, Ijzerman AP, Hill SJ. (2004) Coupling of the human A1 adenosine receptor to different heterotrimeric G proteins: evidence for agonist-specific G protein activation. Br J Pharmacol, 143 (6): 705-14. [PMID:15302686]
22. Crawford M, Ford S, Henry M, Matherne GP, Lankford A. (2005) Myocardial function following cold ischemic storage is improved by cardiac-specific overexpression of A1-adenosine receptors. Can J Physiol Pharmacol, 83 (6): 493-8. [PMID:16049549]
23. Dalpiaz A, Townsend-Nicholson A, Beukers MW, Schofield PR, IJzerman AP. (1998) Thermodynamics of full agonist, partial agonist, and antagonist binding to wild-type and mutant adenosine A1 receptors. Biochem Pharmacol, 56 (11): 1437-45. [PMID:9827575]
24. Daly JW, Hide I, Müller CE, Shamim M. (1991) Caffeine analogs: structure-activity relationships at adenosine receptors. Pharmacology, 42 (6): 309-21. [PMID:1658821]
25. Daly JW, Padgett WL, Secunda SI, Thompson RD, Olsson RA. (1993) Structure-activity relationships for 2-substituted adenosines at A1 and A2 adenosine receptors. Pharmacology, 46 (2): 91-100. [PMID:8441759]
26. de Ligt RA, Rivkees SA, Lorenzen A, Leurs R, IJzerman AP. (2005) A "locked-on," constitutively active mutant of the adenosine A1 receptor. Eur J Pharmacol, 510 (1-2): 1-8. [PMID:15740718]
27. De Lorenzo S, Veggetti M, Muchnik S, Losavio A. (2004) Presynaptic inhibition of spontaneous acetylcholine release induced by adenosine at the mouse neuromuscular junction. Br J Pharmacol, 142 (1): 113-24. [PMID:15066904]
28. Deckert J, Berger W, Kleopa K, Heckers S, Ransmayr G, Heinsen H, Beckmann H, Riederer P. (1993) Adenosine A1 receptors in human hippocampus: inhibition of [3H]8-cyclopentyl-1,3-dipropylxanthine binding by antagonist drugs. Neurosci Lett, 150 (2): 191-4. [PMID:8469419]
29. Dolphin AC, Prestwich SA. (1985) Pertussis toxin reverses adenosine inhibition of neuronal glutamate release. Nature, 316 (6024): 148-50. [PMID:2861569]
30. Dong Q, Ginsberg HN, Erlanger BF. (2001) Overexpression of the A1 adenosine receptor in adipose tissue protects mice from obesity-related insulin resistance. Diabetes Obes Metab, 3 (5): 360-6. [PMID:11703426]
31. Draper-Joyce CJ, Bhola R, Wang J, Bhattarai A, Nguyen ATN, Cowie-Kent I, O'Sullivan K, Chia LY, Venugopal H, Valant C et al.. (2021) Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia. Nature, 597 (7877): 571-576. [PMID:34497422]
32. Draper-Joyce CJ, Khoshouei M, Thal DM, Liang YL, Nguyen ATN, Furness SGB, Venugopal H, Baltos JA, Plitzko JM, Danev R et al.. (2018) Structure of the adenosine-bound human adenosine A1 receptor-Gi complex. Nature, 558 (7711): 559-563. [PMID:29925945]
33. Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. (2005) Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron, 48 (6): 1011-23. [PMID:16364904]
34. Eastwood P, Gonzalez J, Paredes S, Nueda A, Domenech T, Alberti J, Vidal B. (2010) Discovery of N-(5,6-diarylpyridin-2-yl)amide derivatives as potent and selective A(2B) adenosine receptor antagonists. Bioorg Med Chem Lett, 20 (5): 1697-700. [PMID:20137946]
35. Elzein E, Kalla RV, Li X, Perry T, Gimbel A, Zeng D, Lustig D, Leung K, Zablocki J. (2008) Discovery of a novel A2B adenosine receptor antagonist as a clinical candidate for chronic inflammatory airway diseases. J Med Chem, 51 (7): 2267-78. [PMID:18321039]
36. Elzein E, Zablocki J. (2008) A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin Investig Drugs, 17 (12): 1901-10. [PMID:19012505]
37. Feoktistov I, Garland EM, Goldstein AE, Zeng D, Belardinelli L, Wells JN, Biaggioni I. (2001) Inhibition of human mast cell activation with the novel selective adenosine A(2B) receptor antagonist 3-isobutyl-8-pyrrolidinoxanthine (IPDX)(2). Biochem Pharmacol, 62 (9): 1163-73. [PMID:11705449]
38. Franchetti P, Cappellacci L, Vita P, Petrelli R, Lavecchia A, Kachler S, Klotz KN, Marabese I, Luongo L, Maione S et al.. (2009) N6-Cycloalkyl- and N6-bicycloalkyl-C5'(C2')-modified adenosine derivatives as high-affinity and selective agonists at the human A1 adenosine receptor with antinociceptive effects in mice. J Med Chem, 52 (8): 2393-406. [PMID:19317449]
39. Fredholm BB. (1995) Astra Award Lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol, 76 (2): 93-101. [PMID:7746802]
40. Fredholm BB, Chen JF, Masino SA, Vaugeois JM. (2005) Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol, 45: 385-412. [PMID:15822182]
41. Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev, 63 (1): 1-34. [PMID:21303899]
42. Furuta S, Onodera K, Kumagai M, Honma I, Miyazaki S, Sato T, Sakurada S. (2003) Involvement of adenosine A1 receptors in forced walking stress-induced analgesia in mice. Methods Find Exp Clin Pharmacol, 25 (10): 793-6. [PMID:14735226]
43. Gao ZG, Mamedova LK, Chen P, Jacobson KA. (2004) 2-Substituted adenosine derivatives: affinity and efficacy at four subtypes of human adenosine receptors. Biochem Pharmacol, 68 (10): 1985-93. [PMID:15476669]
44. Germack R, Dickenson JM. (2005) Adenosine triggers preconditioning through MEK/ERK1/2 signalling pathway during hypoxia/reoxygenation in neonatal rat cardiomyocytes. J Mol Cell Cardiol, 39 (3): 429-42. [PMID:16005018]
45. Germack R, Griffin M, Dickenson JM. (2004) Activation of protein kinase B by adenosine A1 and A3 receptors in newborn rat cardiomyocytes. J Mol Cell Cardiol, 37 (5): 989-99. [PMID:15522276]
46. Ghanem E, Lövdahl C, Daré E, Ledent C, Fredholm BB, Boeynaems JM, Van Driessche W, Beauwens R. (2005) Luminal adenosine stimulates chloride secretion through A1 receptor in mouse jejunum. Am J Physiol Gastrointest Liver Physiol, 288 (5): G972-7. [PMID:15637180]
47. Giffin NJ, Kowacs F, Libri V, Williams P, Goadsby PJ, Kaube H. (2003) Effect of the adenosine A1 receptor agonist GR79236 on trigeminal nociception with blink reflex recordings in healthy human subjects. Cephalalgia, 23 (4): 287-92. [PMID:12716347]
48. Gillespie RJ, Bamford SJ, Botting R, Comer M, Denny S, Gaur S, Griffin M, Jordan AM, Knight AR, Lerpiniere J et al.. (2009) Antagonists of the human A(2A) adenosine receptor. 4. Design, synthesis, and preclinical evaluation of 7-aryltriazolo[4,5-d]pyrimidines. J Med Chem, 52 (1): 33-47. [PMID:19072055]
49. Giménez-Llort L, Fernández-Teruel A, Escorihuela RM, Fredholm BB, Tobeña A, Pekny M, Johansson B. (2002) Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur J Neurosci, 16: 547-550. [PMID:12193199]
50. Giménez-Llort L, Masino SA, Diao L, Fernández-Teruel A, Tobeña A, Halldner L, Fredholm BB. (2005) Mice lacking the adenosine A1 receptor have normal spatial learning and plasticity in the CA1 region of the hippocampus, but they habituate more slowly. Synapse, 57 (1): 8-16. [PMID:15858837]
51. Glukhova A, Thal DM, Nguyen AT, Vecchio EA, Jörg M, Scammells PJ, May LT, Sexton PM, Christopoulos A. (2017) Structure of the Adenosine A1 Receptor Reveals the Basis for Subtype Selectivity. Cell, 168 (5): 867-877.e13. [PMID:28235198]
52. González-Benítez E, Guinzberg R, Díaz-Cruz A, Piña E. (2002) Regulation of glycogen metabolism in hepatocytes through adenosine receptors. Role of Ca2+ and cAMP. Eur J Pharmacol, 437 (3): 105-11. [PMID:11890897]
53. Grahner B, Winiwarter S, Lanzner W, Müller CE. (1994) Synthesis and structure-activity relationships of deazaxanthines: analogs of potent A1- and A2-adenosine receptor antagonists. J Med Chem, 37 (10): 1526-34. [PMID:8182711]
54. Guo D, Peletier LA, Bridge L, Keur W, de Vries H, Zweemer A, Heitman LH, IJzerman AP. (2018) A two-state model for the kinetics of competitive radioligand binding. Br J Pharmacol, 175 (10): 1719-1730. [PMID:29486053]
55. Hayallah AM, Sandoval-Ramírez J, Reith U, Schobert U, Preiss B, Schumacher B, Daly JW, Müller CE. (2002) 1,8-disubstituted xanthine derivatives: synthesis of potent A2B-selective adenosine receptor antagonists. J Med Chem, 45 (7): 1500-10. [PMID:11906291]
56. Heitman LH, Mulder-Krieger T, Spanjersberg RF, von Frijtag Drabbe Künzel JK, Dalpiaz A, IJzerman AP. (2006) Allosteric modulation, thermodynamics and binding to wild-type and mutant (T277A) adenosine A1 receptors of LUF5831, a novel nonadenosine-like agonist. Br J Pharmacol, 147 (5): 533-41. [PMID:16444290]
57. Hirai H, Okada Y. (1995) Adenosine facilitates in vivo neurotransmission in the superior colliculus of the rat. J Neurophysiol, 74 (3): 950-60. [PMID:7500164]
58. Hoffman AF, Laaris N, Kawamura M, Masino SA, Lupica CR. (2010) Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. J Neurosci, 30 (2): 545-55. [PMID:20071517]
59. Holschbach MH, Olsson RA, Bier D, Wutz W, Sihver W, Schüller M, Palm B, Coenen HH. (2002) Synthesis and evaluation of no-carrier-added 8-cyclopentyl-3-(3-[(18)F]fluoropropyl)-1-propylxanthine ([(18)F]CPFPX): a potent and selective A(1)-adenosine receptor antagonist for in vivo imaging. J Med Chem, 45 (23): 5150-6. [PMID:12408725]
60. Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. (2005) Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci, 8 (7): 858-9. [PMID:15965471]
61. Imlach WL, Bhola RF, May LT, Christopoulos A, Christie MJ. (2015) A Positive Allosteric Modulator of the Adenosine A1 Receptor Selectively Inhibits Primary Afferent Synaptic Transmission in a Neuropathic Pain Model. Mol Pharmacol, 88 (3): 460-8. [PMID:26104547]
62. Iredale PA, Alexander SP, Hill SJ. (1994) Coupling of a transfected human brain A1 adenosine receptor in CHO-K1 cells to calcium mobilisation via a pertussis toxin-sensitive mechanism. Br J Pharmacol, 111 (4): 1252-6. [PMID:8032613]
63. Jacobson KA IJzerman AP, Linden J. (1999) 1,3-Dialkylxanthine derivatives having high potency as antagonists at human A2B adenosine receptors. Drug Dev Res, (47): 45-53.
64. Jacobson KA, Gallo-Rodriguez C, Melman N, Fischer B, Maillard M, van Bergen A, van Galen PJ, Karton Y. (1993) Structure-activity relationships of 8-styrylxanthines as A2-selective adenosine antagonists. J Med Chem, 36 (10): 1333-42. [PMID:8496902]
65. Jacobson KA, Gao ZG. (2006) Adenosine receptors as therapeutic targets. Nat Rev Drug Discov, 5 (3): 247-64. [PMID:16518376]
66. Jockers R, Linder ME, Hohenegger M, Nanoff C, Bertin B, Strosberg AD, Marullo S, Freissmuth M. (1994) Species difference in the G protein selectivity of the human and bovine A1-adenosine receptor. J Biol Chem, 269 (51): 32077-84. [PMID:7798201]
67. Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Giménez-Llort L, Escorihuela RM, Fernández-Teruel A, Wiesenfeld-Hallin Z, Xu XJ et al.. (2001) Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA, 98 (16): 9407-12. [PMID:11470917]
68. Johansson SM, Lindgren E, Yang JN, Herling AW, Fredholm BB. (2008) Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur J Pharmacol, 597 (1-3): 92-101. [PMID:18789919]
69. Johansson SM, Yang JN, Lindgren E, Fredholm BB. (2007) Eliminating the antilipolytic adenosine A1 receptor does not lead to compensatory changes in the antilipolytic actions of PGE2 and nicotinic acid. Acta Physiol (Oxf), 190 (1): 87-96. [PMID:17428236]
70. Kalk P, Eggert B, Relle K, Godes M, Heiden S, Sharkovska Y, Fischer Y, Ziegler D, Bielenberg GW, Hocher B. (2007) The adenosine A1 receptor antagonist SLV320 reduces myocardial fibrosis in rats with 5/6 nephrectomy without affecting blood pressure. Br J Pharmacol, 151 (7): 1025-32. [PMID:17558436]
71. Kara FM, Chitu V, Sloane J, Axelrod M, Fredholm BB, Stanley ER, Cronstein BN. (2010) Adenosine A1 receptors (A1Rs) play a critical role in osteoclast formation and function. FASEB J, 24 (7): 2325-33. [PMID:20181934]
72. Kara FM, Doty SB, Boskey A, Goldring S, Zaidi M, Fredholm BB, Cronstein BN. (2010) Adenosine A(1) receptors regulate bone resorption in mice: adenosine A(1) receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A(1) receptor-knockout mice. Arthritis Rheum, 62 (2): 534-41. [PMID:20112380]
73. Karlsten R, Gordh Jr T, Hartvig P, Post C. (1990) Effects of intrathecal injection of the adenosine receptor agonists R-phenylisopropyl-adenosine and N-ethylcarboxamide-adenosine on nociception and motor function in the rat. Anesth Analg, 71 (1): 60-4. [PMID:2363530]
74. Karton Y, Jiang JL, Ji XD, Melman N, Olah ME, Stiles GL, Jacobson KA. (1996) Synthesis and biological activities of flavonoid derivatives as A3 adenosine receptor antagonists. J Med Chem, 39 (12): 2293-301. [PMID:8691424]
75. Kiesman WF, Zhao J, Conlon PR, Dowling JE, Petter RC, Lutterodt F, Jin X, Smits G, Fure M, Jayaraj A et al.. (2006) Potent and orally bioavailable 8-bicyclo[2.2.2]octylxanthines as adenosine A1 receptor antagonists. J Med Chem, 49 (24): 7119-31. [PMID:17125264]
76. Kim SA, Marshall MA, Melman N, Kim HS, Müller CE, Linden J, Jacobson KA. (2002) Structure-activity relationships at human and rat A2B adenosine receptors of xanthine derivatives substituted at the 1-, 3-, 7-, and 8-positions. J Med Chem, 45 (11): 2131-8. [PMID:12014951]
77. Kim YC, Ji X, Melman N, Linden J, Jacobson KA. (2000) Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors. J Med Chem, 43 (6): 1165-72. [PMID:10737749]
78. Kirchhof P, Fabritz L, Fortmuller L, Matherne GP, Lankford A, Baba HA, Schmitz W, Breithardt G, Neumann J, Boknik P. (2003) Altered sinus nodal and atrioventricular nodal function in freely moving mice overexpressing the A1 adenosine receptor. Am J Physiol Heart Circ Physiol, 285 (1): H145-53. [PMID:12637351]
79. Klotz KN, Falgner N, Kachler S, Lambertucci C, Vittori S, Volpini R, Cristalli G. (2007) [3H]HEMADO--a novel tritiated agonist selective for the human adenosine A3 receptor. Eur J Pharmacol, 556 (1-3): 14-8. [PMID:17126322]
80. Klotz KN, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, Lohse MJ. (1998) Comparative pharmacology of human adenosine receptor subtypes - characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol, 357 (1): 1-9. [PMID:9459566]
81. Klotz KN, Vogt H, Tawfik-Schlieper H. (1991) Comparison of A1 adenosine receptors in brain from different species by radioligand binding and photoaffinity labelling. Naunyn Schmiedebergs Arch Pharmacol, 343 (2): 196-201. [PMID:2067592]
82. Kochanek PM, Vagni VA, Janesko KL, Washington CB, Crumrine PK, Garman RH, Jenkins LW, Clark RS, Homanics GE, Dixon CE et al.. (2006) Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab, 26 (4): 565-75. [PMID:16121125]
83. Kuno A, Critz SD, Cui L, Solodushko V, Yang XM, Krahn T, Albrecht B, Philipp S, Cohen MV, Downey JM. (2007) Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol, 43 (3): 262-71. [PMID:17632123]
84. Lang UE, Lang F, Richter K, Vallon V, Lipp HP, Schnermann J, Wolfer DP. (2003) Emotional instability but intact spatial cognition in adenosine receptor 1 knock out mice. Behav Brain Res, 145 (1-2): 179-88. [PMID:14529816]
85. Lankford AR, Yang JN, Rose'Meyer R, French BA, Matherne GP, Fredholm BB, Yang Z. (2006) Effect of modulating cardiac A1 adenosine receptor expression on protection with ischemic preconditioning. Am J Physiol Heart Circ Physiol, 290 (4): H1469-73. [PMID:16299262]
86. Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. (2004) A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol, 286 (2): F298-306. [PMID:14600029]
87. Liang BT, Urso R, Sambraski E, Jacobson KA. (2010) . In A3 Adenosine Receptors from Cell Biology to Pharmacology and Therapeutics. Edited by Borea PA (Springer) . [ISBN:9789048131440]
88. Libert F, Van Sande J, Lefort A, Czernilofsky A, Dumont JE, Vassart G, Ensinger HA, Mendla KD. (1992) Cloning and functional characterization of a human A1 adenosine receptor. Biochem Biophys Res Commun, 187 (2): 919-26. [PMID:1530647]
89. Liu H, Kuang X, Zhang Y, Ye Y, Li J, Liang L, Xie Z, Weng L, Guo J, Li H et al.. (2020) ADORA1 Inhibition Promotes Tumor Immune Evasion by Regulating the ATF3-PD-L1 Axis. Cancer Cell, 37 (3): 324-339.e8. [PMID:32183950]
90. Lopes LV, Halldner L, Rebola N, Johansson B, Ledent C, Chen JF, Fredholm BB, Cunha RA. (2004) Binding of the prototypical adenosine A(2A) receptor agonist CGS 21680 to the cerebral cortex of adenosine A(1) and A(2A) receptor knockout mice. Br J Pharmacol, 141: 1006-1014. [PMID:14993095]
91. Müller CE. (2000) A2A Adenosine receptor antagonists—future drugs for Parkinson’s disease?. Drugs Future, (25): 1043-1052.
92. Müller CE, Stein B. (1996) Adenosine receptor antagonists: structures and potential therapeutic applications. Curr Pharm Des, 2: 501-530.
93. Ma HC, Wang YF, Feng CS, Zhao H, Dohi S. (2005) Effects of adenosine agonist R-phenylisopropyl-adenosine on halothane anesthesia and antinociception in rats. Acta Pharmacol Sin, 26 (2): 181-5. [PMID:15663896]
94. MacGregor DG, Miller WJ, Stone TW. (1993) Mediation of the neuroprotective action of R-phenylisopropyl-adenosine through a centrally located adenosine A1 receptor. Br J Pharmacol, 110 (1): 470-6. [PMID:8220909]
95. Maemoto T, Tada M, Mihara T, Ueyama N, Matsuoka H, Harada K, Yamaji T, Shirakawa K, Kuroda S, Akahane A et al.. (2004) Pharmacological characterization of FR194921, a new potent, selective, and orally active antagonist for central adenosine A1 receptors. J Pharmacol Sci, 96 (1): 42-52. [PMID:15351792]
96. Mahan LC, McVittie LD, Smyk-Randall EM, Nakata H, Monsma Jr FJ, Gerfen CR, Sibley DR. (1991) Cloning and expression of an A1 adenosine receptor from rat brain. Mol Pharmacol, 40 (1): 1-7. [PMID:1857334]
97. Marquardt DL, Walker LL, Heinemann S. (1994) Cloning of two adenosine receptor subtypes from mouse bone marrow-derived mast cells. J Immunol, 152 (9): 4508-15. [PMID:8157966]
98. Martin PL, Wysocki Jr RJ, Barrett RJ, May JM, Linden J. (1996) Characterization of 8-(N-methylisopropyl)amino-N6-(5'-endohydroxy- endonorbornyl)-9-methyladenine (WRC-0571), a highly potent and selective, non-xanthine antagonist of A1 adenosine receptors. J Pharmacol Exp Ther, 276 (2): 490-9. [PMID:8632314]
99. Matherne GP, Linden J, Byford AM, Gauthier NS, Headrick JP. (1997) Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proc Natl Acad Sci USA, 94 (12): 6541-6. [PMID:9177254]
100. Meibom D, Albrecht-Küpper B, Diedrichs N, Hübsch W, Kast R, Krämer T, Krenz U, Lerchen HG, Mittendorf J, Nell PG et al.. (2017) Neladenoson Bialanate Hydrochloride: A Prodrug of a Partial Adenosine A1 Receptor Agonist for the Chronic Treatment of Heart Diseases. ChemMedChem, 12 (10): 728-737. [PMID:28488817]
101. Melman A, Gao ZG, Kumar D, Wan TC, Gizewski E, Auchampach JA, Jacobson KA. (2008) Design of (N)-methanocarba adenosine 5'-uronamides as species-independent A3 receptor-selective agonists. Bioorg Med Chem Lett, 18 (9): 2813-9. [PMID:18424135]
102. Minelli A, Allegrucci C, Piomboni P, Mannucci R, Lluis C, Franco R. (2000) Immunolocalization of A1 adenosine receptors in mammalian spermatozoa. J Histochem Cytochem, 48 (9): 1163-71. [PMID:10950874]
103. Minelli A, Liguori L, Bellazza I, Mannucci R, Johansson B, Fredholm BB. (2004) Involvement of A1 adenosine receptors in the acquisition of fertilizing capacity. J Androl, 25 (2): 286-92. [PMID:14760015]
104. Minetti P, Tinti MO, Carminati P, Castorina M, Di Cesare MA, Di Serio S, Gallo G, Ghirardi O, Giorgi F, Giorgi L et al.. (2005) 2-n-Butyl-9-methyl-8-[1,2,3]triazol-2-yl-9H-purin-6-ylamine and analogues as A2A adenosine receptor antagonists. Design, synthesis, and pharmacological characterization. J Med Chem, 48 (22): 6887-96. [PMID:16250647]
105. Murray RD, Churchill PC. (1984) Effects of adenosine receptor agonists in the isolated, perfused rat kidney. Am J Physiol, 247 (3 Pt 2): H343-8. [PMID:6089592]
106. Müller CE, Ferré S. (2007) Blocking striatal adenosine A2A receptors: a new strategy for basal ganglia disorders. Recent Pat CNS Drug Discov, 2 (1): 1-21. [PMID:18221214]
107. Müller CE, Jacobson KA. (2011) Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim Biophys Acta, 1808 (5): 1290-308. [PMID:21185259]
108. Müller CE, Shi D, Manning M, Daly JW. (1993) Synthesis of paraxanthine analogs (1,7-disubstituted xanthines) and other xanthines unsubstituted at the 3-position: structure-activity relationships at adenosine receptors. J Med Chem, 36 (22): 3341-9. [PMID:8230124]
109. Müller CE, Thorand M, Qurishi R, Diekmann M, Jacobson KA, Padgett WL, Daly JW. (2002) Imidazo[2,1-i]purin-5-ones and related tricyclic water-soluble purine derivatives: potent A(2A)- and A(3)-adenosine receptor antagonists. J Med Chem, 45 (16): 3440-50. [PMID:12139454]
110. Nguyen AT, Vecchio EA, Thomas T, Nguyen TD, Aurelio L, Scammells PJ, White PJ, Sexton PM, Gregory KJ, May LT et al.. (2016) Role of the Second Extracellular Loop of the Adenosine A1 Receptor on Allosteric Modulator Binding, Signaling, and Cooperativity. Mol Pharmacol, 90 (6): 715-725. [PMID:27683013]
111. Nowak M, Lynch L, Yue S, Ohta A, Sitkovsky M, Balk SP, Exley MA. (2010) The A2aR adenosine receptor controls cytokine production in iNKT cells. Eur J Immunol, 40 (3): 682-7. [PMID:20039304]
112. O'Shaughnessy CT, Aram JA, Lodge D. (1988) A1 adenosine receptor-mediated block of epileptiform activity induced in zero magnesium in rat neocortex in vitro. Epilepsy Res, 2 (5): 294-301. [PMID:2461856]
113. Obiefuna PC, Batra VK, Nadeem A, Borron P, Wilson CN, Mustafa SJ. (2005) A novel A1 adenosine receptor antagonist, L-97-1 [3-[2-(4-aminophenyl)-ethyl]-8-benzyl-7-{2-ethyl-(2-hydroxy-ethyl)-amino]-ethyl}-1-propyl-3,7-dihydro-purine-2,6-dione], reduces allergic responses to house dust mite in an allergic rabbit model of asthma. J Pharmacol Exp Ther, 315 (1): 329-36. [PMID:16020631]
114. Olsson T, Cronberg T, Rytter A, Asztély F, Fredholm BB, Smith ML, Wieloch T. (2004) Deletion of the adenosine A1 receptor gene does not alter neuronal damage following ischaemia in vivo or in vitro. Eur J Neurosci, 20 (5): 1197-204. [PMID:15341591]
115. Ongini E, Dionisotti S, Gessi S, Irenius E, Fredholm BB. (1999) Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol, 359 (1): 7-10. [PMID:9933143]
116. Ozola V, Thorand M, Diekmann M, Qurishi R, Schumacher B, Jacobson KA, Müller CE. (2003) 2-Phenylimidazo[2,1-i]purin-5-ones: structure-activity relationships and characterization of potent and selective inverse agonists at Human A3 adenosine receptors. Bioorg Med Chem, 11 (3): 347-56. [PMID:12517430]
117. Pfeifer CA, Suzuki F, Jackson EK. (1995) Selective A1 adenosine receptor antagonism augments beta-adrenergic-induced renin release in vivo. Am J Physiol, 269 (4 Pt 2): F469-79. [PMID:7485531]
118. Pfister JR, Belardinelli L, Lee G, Lum RT, Milner P, Stanley WC, Linden J, Baker SP, Schreiner G. (1997) Synthesis and biological evaluation of the enantiomers of the potent and selective A1-adenosine antagonist 1,3-dipropyl-8-[2-(5,6-epoxynorbonyl)]-xanthine. J Med Chem, 40 (12): 1773-8. [PMID:9191953]
119. Poon A, Sawynok J. (1998) Antinociception by adenosine analogs and inhibitors of adenosine metabolism in an inflammatory thermal hyperalgesia model in the rat. Pain, 74 (2-3): 235-45. [PMID:9520238]
120. Ren H, Stiles GL. (1994) Characterization of the human A1 adenosine receptor gene. Evidence for alternative splicing. J Biol Chem, 269 (4): 3104-10. [PMID:8300646]
121. Rice AM, Fain JN, Rivkees SA. (2000) A1 adenosine receptor activation increases adipocyte leptin secretion. Endocrinology, 141 (4): 1442-5. [PMID:10746648]
122. Rivkees SA. (1994) Localization and characterization of adenosine receptor expression in rat testis. Endocrinology, 135 (6): 2307-13. [PMID:7988413]
123. Rivkees SA, Barbhaiya H, IJzerman AP. (1999) Identification of the adenine binding site of the human A1 adenosine receptor. J Biol Chem, 274 (6): 3617-21. [PMID:9920910]
124. Saki M, Tsumuki H, Nonaka H, Shimada J, Ichimura M. (2002) KF26777 (2-(4-bromophenyl)-7,8-dihydro-4-propyl-1H-imidazo[2,1-i]purin-5(4H)-one dihydrochloride), a new potent and selective adenosine A3 receptor antagonist. Eur J Pharmacol, 444 (3): 133-41. [PMID:12063073]
125. Salehi A, Parandeh F, Fredholm BB, Grapengiesser E, Hellman B. (2009) Absence of adenosine A1 receptors unmasks pulses of insulin release and prolongs those of glucagon and somatostatin. Life Sci, 85 (11-12): 470-6. [PMID:19682463]
126. Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. (1993) Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci USA, 90 (21): 10365-9. [PMID:8234299]
127. Satoh A, Shimosegawa T, Satoh K, Ito H, Kohno Y, Masamune A, Fujita M, Toyota T. (2000) Activation of adenosine A1-receptor pathway induces edema formation in the pancreas of rats. Gastroenterology, 119 (3): 829-36. [PMID:10982777]
128. Sauer R, Maurinsh J, Reith U, Fülle F, Klotz KN, Müller CE. (2000) Water-soluble phosphate prodrugs of 1-propargyl-8-styrylxanthine derivatives, A(2A)-selective adenosine receptor antagonists. J Med Chem, 43 (3): 440-8. [PMID:10669571]
129. Scammell TE, Arrigoni E, Thompson MA, Ronan PJ, Saper CB, Greene RW. (2003) Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. J Neurosci, 23 (13): 5762-70. [PMID:12843280]
130. Schindler M, Harris CA, Hayes B, Papotti M, Humphrey PP. (2001) Immunohistochemical localization of adenosine A1 receptors in human brain regions. Neurosci Lett, 297 (3): 211-5. [PMID:11137765]
131. Schnermann J, Weihprecht H, Briggs JP. (1990) Inhibition of tubuloglomerular feedback during adenosine1 receptor blockade. Am J Physiol, 258 (3 Pt 2): F553-61. [PMID:1969237]
132. Schnurr M, Toy T, Shin A, Hartmann G, Rothenfusser S, Soellner J, Davis ID, Cebon J, Maraskovsky E. (2004) Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells. Blood, 103 (4): 1391-7. [PMID:14551144]
133. Scholz KP, Miller RJ. (1992) Inhibition of quantal transmitter release in the absence of calcium influx by a G protein-linked adenosine receptor at hippocampal synapses. Neuron, 8 (6): 1139-50. [PMID:1351733]
134. Schulte G, Fredholm BB. (2000) Human adenosine A(1), A(2A), A(2B), and A(3) receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol Pharmacol, 58 (3): 477-82. [PMID:10953039]
135. Schulte G, Robertson B, Fredholm BB, DeLander GE, Shortland P, Molander C. (2003) Distribution of antinociceptive adenosine A1 receptors in the spinal cord dorsal horn, and relationship to primary afferents and neuronal subpopulations. Neuroscience, 121 (4): 907-16. [PMID:14580941]
136. Schweda F, Segerer F, Castrop H, Schnermann J, Kurtz A. (2005) Blood pressure-dependent inhibition of Renin secretion requires A1 adenosine receptors. Hypertension, 46 (4): 780-6. [PMID:16172432]
137. Sjölund KF, Sollevi A, Segerdahl M, Lundeberg T. (1997) Intrathecal adenosine analog administration reduces substance P in cerebrospinal fluid along with behavioral effects that suggest antinociception in rats. Anesth Analg, 85 (3): 627-32. [PMID:9296420]
138. Stenberg D, Litonius E, Halldner L, Johansson B, Fredholm BB, Porkka-Heiskanen T. (2003) Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J Sleep Res, 12 (4): 283-90. [PMID:14633239]
139. Storr M, Thammer J, Dunkel R, Schusdziarra V, Allescher HD. (2002) Modulatory effect of adenosine receptors on the ascending and descending neural reflex responses of rat ileum. BMC Neurosci, 3: 21. [PMID:12495441]
140. Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. (2001) Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA, 98 (17): 9983-8. [PMID:11504952]
141. Thomson S, Bao D, Deng A, Vallon V. (2000) Adenosine formed by 5'-nucleotidase mediates tubuloglomerular feedback. J Clin Invest, 106 (2): 289-98. [PMID:10903345]
142. Tosh DK, Rao H, Bitant A, Salmaso V, Mannes P, Lieberman DI, Vaughan KL, Mattison JA, Rothwell AC, Auchampach JA et al.. (2019) Design and in Vivo Characterization of A1 Adenosine Receptor Agonists in the Native Ribose and Conformationally Constrained (N)-Methanocarba Series. J Med Chem, 62 (3): 1502-1522. [PMID:30605331]
143. Townsend-Nicholson A, Schofield PR. (1994) A threonine residue in the seventh transmembrane domain of the human A1 adenosine receptor mediates specific agonist binding. J Biol Chem, 269 (4): 2373-6. [PMID:8300561]
144. Townsend-Nicholson A, Shine J. (1992) Molecular cloning and characterisation of a human brain A1 adenosine receptor cDNA. Brain Res Mol Brain Res, 16 (3-4): 365-70. [PMID:1339301]
145. Turner CP, Seli M, Ment L, Stewart W, Yan H, Johansson B, Fredholm BB, Blackburn M, Rivkees SA. (2003) A1 adenosine receptors mediate hypoxia-induced ventriculomegaly. Proc Natl Acad Sci USA, 100 (20): 11718-22. [PMID:12975523]
146. van Muijlwijk-Koezen JE, Timmerman H, Link R, van der Goot H, IJzerman AP. (1998) A novel class of adenosine A3 receptor ligands. 1. 3-(2-Pyridinyl)isoquinoline derivatives. J Med Chem, 41 (21): 3987-93. [PMID:9767636]
147. Varani K, Gessi S, Merighi S, Vincenzi F, Cattabriga E, Benini A, Klotz KN, Baraldi PG, Tabrizi MA, Lennan SM et al.. (2005) Pharmacological characterization of novel adenosine ligands in recombinant and native human A2B receptors. Biochem Pharmacol, 70 (11): 1601-12. [PMID:16219300]
148. Varani K, Merighi S, Gessi S, Klotz KN, Leung E, Baraldi PG, Cacciari B, Romagnoli R, Spalluto G, Borea PA. (2000) [(3)H]MRE 3008F20: a novel antagonist radioligand for the pharmacological and biochemical characterization of human A(3) adenosine receptors. Mol Pharmacol, 57 (5): 968-75. [PMID:10779381]
149. Vidal B, Nueda A, Esteve C, Domenech T, Benito S, Reinoso RF, Pont M, Calbet M, López R, Cadavid MI et al.. (2007) Discovery and characterization of 4'-(2-furyl)-N-pyridin-3-yl-4,5'-bipyrimidin-2'-amine (LAS38096), a potent, selective, and efficacious A2B adenosine receptor antagonist. J Med Chem, 50 (11): 2732-6. [PMID:17469811]
150. Vitzthum H, Weiss B, Bachleitner W, Krämer BK, Kurtz A. (2004) Gene expression of adenosine receptors along the nephron. Kidney Int, 65 (4): 1180-90. [PMID:15086457]
151. Volpini R, Costanzi S, Lambertucci C, Taffi S, Vittori S, Klotz KN, Cristalli G. (2002) N(6)-alkyl-2-alkynyl derivatives of adenosine as potent and selective agonists at the human adenosine A(3) receptor and a starting point for searching A(2B) ligands. J Med Chem, 45 (15): 3271-9. [PMID:12109910]
152. Voors AA, Bax JJ, Hernandez AF, Wirtz AB, Pap AF, Ferreira AC, Senni M, van der Laan M, Butler J, PANTHEON Investigators. (2019) Safety and efficacy of the partial adenosine A1 receptor agonist neladenoson bialanate in patients with chronic heart failure with reduced ejection fraction: a phase IIb, randomized, double-blind, placebo-controlled trial. Eur J Heart Fail, 21 (11): 1426-1433. [PMID:31523892]
153. Weiss SM, Benwell K, Cliffe IA, Gillespie RJ, Knight AR, Lerpiniere J, Misra A, Pratt RM, Revell D, Upton R et al.. (2003) Discovery of nonxanthine adenosine A2A receptor antagonists for the treatment of Parkinson's disease. Neurology, 61 (11 Suppl 6): S101-6. [PMID:14663021]
154. Weyler S, Fülle F, Diekmann M, Schumacher B, Hinz S, Klotz KN, Müller CE. (2006) Improving potency, selectivity, and water solubility of adenosine A1 receptor antagonists: xanthines modified at position 3 and related pyrimido[1,2,3-cd]purinediones. ChemMedChem, 1 (8): 891-902. [PMID:16902942]
155. Wittendorp MC, von Frijtag Drabbe Künzel J, Ijzerman AP, Boddeke HW, Biber K. (2004) The mouse brain adenosine A1 receptor: functional expression and pharmacology. Eur J Pharmacol, 487 (1-3): 73-9. [PMID:15033378]
156. Wu WP, Hao JX, Halldner L, Lövdahl C, DeLander GE, Wiesenfeld-Hallin Z, Fredholm BB, Xu XJ. (2005) Increased nociceptive response in mice lacking the adenosine A1 receptor. Pain, 113 (3): 395-404. [PMID:15661449]
157. Yamamoto S, Nakanishi O, Matsui T, Shinohara N, Kinoshita H, Lambert C, Ishikawa T. (2003) Intrathecal adenosine A1 receptor agonist attenuates hyperalgesia without inhibiting spinal glutamate release in the rat. Cell Mol Neurobiol, 23 (2): 175-85. [PMID:12735630]
158. Yan L, Burbiel JC, Maass A, Müller CE. (2003) Adenosine receptor agonists: from basic medicinal chemistry to clinical development. Expert Opin Emerg Drugs, 8 (2): 537-76. [PMID:14662005]
159. Yang JN, Tiselius C, Daré E, Johansson B, Valen G, Fredholm BB. (2007) Sex differences in mouse heart rate and body temperature and in their regulation by adenosine A1 receptors. Acta Physiol (Oxf), 190 (1): 63-75. [PMID:17428234]
160. Yang X, Heitman LH, IJzerman AP, van der Es D. (2021) Molecular probes for the human adenosine receptors. Purinergic Signal, 17 (1): 85-108. [PMID:33313997]
161. Yang X, van Veldhoven JPD, Offringa J, Kuiper BJ, Lenselink EB, Heitman LH, van der Es D, IJzerman AP. (2019) Development of Covalent Ligands for G Protein-Coupled Receptors: A Case for the Human Adenosine A3 Receptor. J Med Chem, 62 (7): 3539-3552. DOI: 10.1021/acs.jmedchem.8b02026 [PMID:30869893]