
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Gene and Protein Information  |
|||||||
| Species | TM | P Loops | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 24 | 4 | 1873 | 1q32.1 | CACNA1S | calcium voltage-gated channel subunit alpha1 S | 14 |
| Mouse | 24 | 4 | 1880 | 1 59.55 cM | Cacna1s | calcium channel, voltage-dependent, L type, alpha 1S subunit | 8 |
| Rat | 24 | 4 | 1850 | 13 | Cacna1s | calcium voltage-gated channel subunit alpha1 S | 10 |
Database Links  |
|
| Alphafold | Q13698 (Hs), Q02789 (Mm), Q02485 (Rn) |
| ChEMBL Target | CHEMBL3805 (Hs), CHEMBL4108 (Rn) |
| DrugBank Target | Q13698 (Hs) |
| Ensembl Gene | ENSG00000081248 (Hs), ENSMUSG00000026407 (Mm), ENSRNOG00000046231 (Rn) |
| Entrez Gene | 779 (Hs), 12292 (Mm), 682930 (Rn) |
| Human Protein Atlas | ENSG00000081248 (Hs) |
| KEGG Gene | hsa:779 (Hs), mmu:12292 (Mm), rno:682930 (Rn) |
| OMIM | 114208 (Hs) |
| Orphanet | ORPHA119157 (Hs) |
| Pharos | Q13698 (Hs) |
| RefSeq Nucleotide | NM_000069 (Hs), NM_014193 (Mm), NM_001081023 (Mm), NM_053873 (Rn) |
| RefSeq Protein | NP_000060 (Hs), NP_001074492 (Mm), NP_055008 (Mm), NP_446325 (Rn) |
| UniProtKB | Q13698 (Hs), Q02789 (Mm), Q02485 (Rn) |
| Wikipedia | CACNA1S (Hs) |
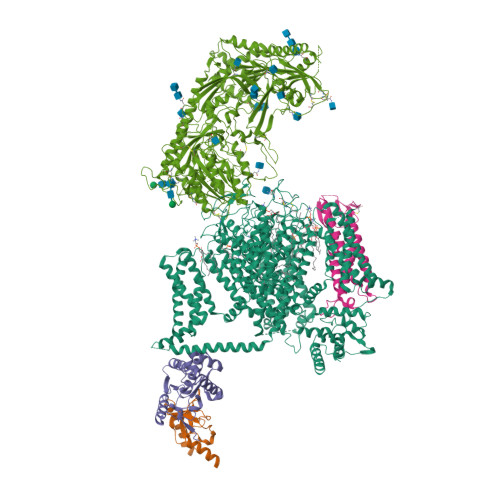
Selected 3D Structures  |
|||||||||||
|
|
||||||||||
Associated Proteins  |
||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||
Functional Characteristics  |
|
| L-type calcium current: High voltage-activated, very slow voltage dependent inactivation | |
Ion Selectivity and Conductance  |
||||||
|
Voltage Dependence  |
||||||||||||||||||||||
|
||||||||||||||||||||||
Download all structure-activity data for this target as a CSV file 
| Activators | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific activator tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Activator Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BAYK 8644 stimulates currents at μM concentrations but, unlike inhibitory dihydropyridines, does not block charge movement [22,32,40]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Gating inhibitors 
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific gating inhibitor tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Channel Blockers | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific channel blocker tables | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Channel Blocker Comments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amlodopine, isradipine, nifedipine, nitrendipine, and nimodipine are examples of dihydropyridine calcium channel antagonists. Verapamil is a phenylalkylamine calcium channel blocker, and diltiazem is a benzothiazepine calcium channel blocker. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
| Tissue Distribution Comments | ||||||||
| On the protein level Cav1.1 channel expression was demonstrated only in skeletal muscle and mRNA expression has been described by one research group in rat and human brain [48], but these findings have not been reproduced by other groups so far [45]. | ||||||||
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
| Physiological Functions Comments | ||||||||
| ECC is absent from dysgenic skeletal muscle cells lacking dihydropyridine receptors (Cav1.1 α1 subunits) and is restored by transfection of these cells with Cav1.1 α1 subunits [44,49]; fast voltage sensor movement of channel triggers opening of ryanodine receptor-mediated calcium release; channel pore opens only slowly and role of calcium entry for physiological function is not clear. | ||||||||
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinically-Relevant Mutations and Pathophysiology Comments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For details of the mechanism of action of CACNA1S mutations see references [6,30,46]. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Biologically Significant Variants 
|
||||||||||||
|
||||||||||||
|
1. Baur CP, Klingler W, Jurkat-Rott K, Froeba G, Schoch E, Marx T, Georgieff M, Lehmann-Horn F. (2000) Xenon does not induce contracture in human malignant hyperthermia muscle. Br J Anaesth, 85 (5): 712-6. [PMID:11094586]
2. Beam KG, Knudson CM. (1988) Calcium currents in embryonic and neonatal mammalian skeletal muscle. J Gen Physiol, 91 (6): 781-98. [PMID:2458429]
3. Beam KG, Knudson CM. (1988) Effect of postnatal development on calcium currents and slow charge movement in mammalian skeletal muscle. J Gen Physiol, 91 (6): 799-815. [PMID:2458430]
4. Caciotti A, Morrone A, Domenici R, Donati MA, Zammarchi E. (2003) Severe prognosis in a large family with hypokalemic periodic paralysis. Muscle Nerve, 27 (2): 165-9. [PMID:12548523]
5. Carpenter D, Ringrose C, Leo V, Morris A, Robinson RL, Halsall PJ, Hopkins PM, Shaw MA. (2009) The role of CACNA1S in predisposition to malignant hyperthermia. BMC Med Genet, 10: 104. [PMID:19825159]
6. Catterall WA. (2010) Ion channel voltage sensors: structure, function, and pathophysiology. Neuron, 67 (6): 915-28. [PMID:20869590]
7. Chabrier S, Monnier N, Lunardi J. (2008) Early onset of hypokalaemic periodic paralysis caused by a novel mutation of the CACNA1S gene. J Med Genet, 45 (10): 686-8. [PMID:18835861]
8. Chaudhari N. (1992) A single nucleotide deletion in the skeletal muscle-specific calcium channel transcript of muscular dysgenesis (mdg) mice. J Biol Chem, 267 (36): 25636-9. [PMID:1281468]
9. Chen F, Liu Y, Sugiura Y, Allen PD, Gregg RG, Lin W. (2011) Neuromuscular synaptic patterning requires the function of skeletal muscle dihydropyridine receptors. Nat Neurosci, 14 (5): 570-7. [PMID:21441923]
10. Chin H, Krall M, Kim HL, Kozak CA, Mock B. (1992) The gene for the alpha 1 subunit of the skeletal muscle dihydropyridine-sensitive calcium channel (Cchl1a3) maps to mouse chromosome 1. Genomics, 14 (4): 1089-91. [PMID:1335956]
11. Desnuelle C, Liot D, Serratrice G, Lombet A. (1985) Biochemical characterization of plasma membrane isolated from human skeletal muscle. FEBS Lett, 188 (2): 222-6. [PMID:2411596]
12. Dirksen RT, Beam KG. (1995) Single calcium channel behavior in native skeletal muscle. J Gen Physiol, 105 (2): 227-47. [PMID:7539048]
13. Dirksen RT, Nakai J, Gonzalez A, Imoto K, Beam KG. (1997) The S5-S6 linker of repeat I is a critical determinant of L-type Ca2+ channel conductance. Biophys J, 73 (3): 1402-9. [PMID:9284307]
14. Drouet B, Garcia L, Simon-Chazottes D, Mattei MG, Guénet JL, Schwartz A, Varadi G, Pinçon-Raymond M. (1993) The gene coding for the alpha 1 subunit of the skeletal dihydropyridine receptor (Cchl1a3 = mdg) maps to mouse chromosome 1 and human 1q32. Mamm Genome, 4 (9): 499-503. [PMID:8118099]
15. Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A. (1988) Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science, 241 (4873): 1661-4. [PMID:2458626]
16. Fan C, Lehmann-Horn F, Weber MA, Bednarz M, Groome JR, Jonsson MK, Jurkat-Rott K. (2013) Transient compartment-like syndrome and normokalaemic periodic paralysis due to a Cav1.1 mutation. Brain, 136 (Pt 12): 3775-86. [PMID:24240197]
17. Flucher BE, Andrews SB, Fleischer S, Marks AR, Caswell A, Powell JA. (1993) Triad formation: organization and function of the sarcoplasmic reticulum calcium release channel and triadin in normal and dysgenic muscle in vitro. J Cell Biol, 123 (5): 1161-74. [PMID:8245124]
18. Flucher BE, Campiglio M. (2019) STAC proteins: The missing link in skeletal muscle EC coupling and new regulators of calcium channel function. Biochim Biophys Acta Mol Cell Res, 1866 (7): 1101-1110. [PMID:30543836]
19. Flucher BE, Tuluc P. (2011) A new L-type calcium channel isoform required for normal patterning of the developing neuromuscular junction. Channels (Austin), 5 (6): 518-24. [PMID:21993196]
20. Fouad G, Dalakas M, Servidei S, Mendell JR, Van den Bergh P, Angelini C, Alderson K, Griggs RC, Tawil R, Gregg R et al.. (1997) Genotype-phenotype correlations of DHP receptor alpha 1-subunit gene mutations causing hypokalemic periodic paralysis. Neuromuscul Disord, 7 (1): 33-8. [PMID:9132138]
21. Freise D, Held B, Wissenbach U, Pfeifer A, Trost C, Himmerkus N, Schweig U, Freichel M, Biel M, Hofmann F et al.. (2000) Absence of the gamma subunit of the skeletal muscle dihydropyridine receptor increases L-type Ca2+ currents and alters channel inactivation properties. J Biol Chem, 275 (19): 14476-81. [PMID:10799530]
22. Glossmann H, Striessnig J. (1990) Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol, 114: 1-105. [PMID:2155469]
23. Goll A, Ferry DR, Striessnig J, Schober M, Glossmann H. (1984) (-)-[3H]Desmethoxyverapamil, a novel Ca2+ channel probe. Binding characteristics and target size analysis of its receptor in skeletal muscle. FEBS Lett, 176 (2): 371-7. [PMID:6092142]
24. Hagiwara M, Adachi-Akahane S, Nagao T. (1997) High-affinity binding of DTZ323, a novel derivative of diltiazem, to rabbit skeletal muscle L-type Ca++ channels. J Pharmacol Exp Ther, 281 (1): 173-9. [PMID:9103495]
25. Hirano M, Kokunai Y, Nagai A, Nakamura Y, Saigoh K, Kusunoki S, Takahashi MP. (2011) A novel mutation in the calcium channel gene in a family with hypokalemic periodic paralysis. J Neurol Sci, 309 (1-2): 9-11. [PMID:21855088]
26. Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. (2002) A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J Biol Chem, 277 (6): 4079-87. [PMID:11733497]
27. Jay SD, Ellis SB, McCue AF, Williams ME, Vedvick TS, Harpold MM, Campbell KP. (1990) Primary structure of the gamma subunit of the DHP-sensitive calcium channel from skeletal muscle. Science, 248 (4954): 490-2. [PMID:2158672]
28. Jurkat-Rott K, Lehmann-Horn F, Elbaz A, Heine R, Gregg RG, Hogan K, Powers PA, Lapie P, Vale-Santos JE, Weissenbach J et al.. (1994) A calcium channel mutation causing hypokalemic periodic paralysis. Hum Mol Genet, 3 (8): 1415-9. [PMID:7987325]
29. Jurkat-Rott K, Lerche H, Lehmann-Horn F. (2002) Skeletal muscle channelopathies. J Neurol, 249 (11): 1493-502. [PMID:12420087]
30. Jurkat-Rott K, Weber MA, Fauler M, Guo XH, Holzherr BD, Paczulla A, Nordsborg N, Joechle W, Lehmann-Horn F. (2009) K+-dependent paradoxical membrane depolarization and Na+ overload, major and reversible contributors to weakness by ion channel leaks. Proc Natl Acad Sci USA, 106 (10): 4036-41. [PMID:19225109]
31. Ke T, Gomez CR, Mateus HE, Castano JA, Wang QK. (2009) Novel CACNA1S mutation causes autosomal dominant hypokalemic periodic paralysis in a South American family. J Hum Genet, 54 (11): 660-4. [PMID:19779499]
32. Lamb GD, Walsh T. (1987) Calcium currents, charge movement and dihydropyridine binding in fast- and slow-twitch muscles of rat and rabbit. J Physiol (Lond.), 393: 595-617. [PMID:2451745]
33. Li FF, Li QQ, Tan ZX, Zhang SY, Liu J, Zhao EY, Yu GC, Zhou J, Zhang LM, Liu SL. (2012) A novel mutation in CACNA1S gene associated with hypokalemic periodic paralysis which has a gender difference in the penetrance. J Mol Neurosci, 46 (2): 378-83. [PMID:21845430]
34. Matthews E, Labrum R, Sweeney MG, Sud R, Haworth A, Chinnery PF, Meola G, Schorge S, Kullmann DM, Davis MB et al.. (2009) Voltage sensor charge loss accounts for most cases of hypokalemic periodic paralysis. Neurology, 72 (18): 1544-7. [PMID:19118277]
35. Matza D, Badou A, Kobayashi KS, Goldsmith-Pestana K, Masuda Y, Komuro A, McMahon-Pratt D, Marchesi VT, Flavell RA. (2008) A scaffold protein, AHNAK1, is required for calcium signaling during T cell activation. Immunity, 28 (1): 64-74. [PMID:18191595]
36. Monnier N, Procaccio V, Stieglitz P, Lunardi J. (1997) Malignant-hyperthermia susceptibility is associated with a mutation of the alpha 1-subunit of the human dihydropyridine-sensitive L-type voltage-dependent calcium-channel receptor in skeletal muscle. Am J Hum Genet, 60 (6): 1316-25. [PMID:9199552]
37. Obermair GJ, Kugler G, Baumgartner S, Tuluc P, Grabner M, Flucher BE. (2005) The Ca2+ channel alpha2delta-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of alpha1S or excitation-contraction coupling. J Biol Chem, 280 (3): 2229-37. [PMID:15536090]
38. Ogawa T, Kashiwagi A, Kikkawa R, Shigeta Y. (1995) Increase of voltage-sensitive calcium channels and calcium accumulation in skeletal muscles of streptozocin-induced diabetic rats. Metab Clin Exp, 44 (11): 1455-61. [PMID:7476334]
39. Pirone A, Schredelseker J, Tuluc P, Gravino E, Fortunato G, Flucher BE, Carsana A, Salvatore F, Grabner M. (2010) Identification and functional characterization of malignant hyperthermia mutation T1354S in the outer pore of the Cavalpha1S-subunit. Am J Physiol, Cell Physiol, 299 (6): C1345-54. [PMID:20861472]
40. Pizarro G, Fitts R, Uribe I, Ríos E. (1989) The voltage sensor of excitation-contraction coupling in skeletal muscle. Ion dependence and selectivity. J Gen Physiol, 94 (3): 405-28. [PMID:2481710]
41. Prinz H, Striessnig J. (1993) Ligand-induced accelerated dissociation of (+)-cis-diltiazem from L-type Ca2+ channels is simply explained by competition for individual attachment points. J Biol Chem, 268 (25): 18580-5. [PMID:8395510]
42. Ptácek LJ, Tawil R, Griggs RC, Engel AG, Layzer RB, Kwieciński H, McManis PG, Santiago L, Moore M, Fouad G et al.. (1994) Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell, 77 (6): 863-8. [PMID:8004673]
43. Ruth P, Röhrkasten A, Biel M, Bosse E, Regulla S, Meyer HE, Flockerzi V, Hofmann F. (1989) Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science, 245 (4922): 1115-8. [PMID:2549640]
44. Ríos E, Pizarro G, Stefani E. (1992) Charge movement and the nature of signal transduction in skeletal muscle excitation-contraction coupling. Annu Rev Physiol, 54: 109-33. [PMID:1562172]
45. Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Hoda JC, Sartori SB, Striessnig J. (2009) Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol, 75 (2): 407-14. [PMID:19029287]
46. Striessnig J, Bolz HJ, Koschak A. (2010) Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels. Pflugers Arch, 460 (2): 361-74. [PMID:20213496]
47. Striessnig J, Hoda JC, Koschak A, Zaghetto F, Müllner C, Sinnegger-Brauns MJ, Wild C, Watschinger K, Trockenbacher A, Pelster G. (2004) L-type Ca2+ channels in Ca2+ channelopathies. Biochem Biophys Res Commun, 322 (4): 1341-6. [PMID:15336981]
48. Takahashi Y, Jeong SY, Ogata K, Goto J, Hashida H, Isahara K, Uchiyama Y, Kanazawa I. (2003) Human skeletal muscle calcium channel alpha1S is expressed in the basal ganglia: distinctive expression pattern among L-type Ca2+ channels. Neurosci Res, 45 (1): 129-37. [PMID:12507731]
49. Tanabe T, Beam KG, Powell JA, Numa S. (1988) Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature, 336 (6195): 134-9. [PMID:2903448]
50. Tang ZZ, Yarotskyy V, Wei L, Sobczak K, Nakamori M, Eichinger K, Moxley RT, Dirksen RT, Thornton CA. (2012) Muscle weakness in myotonic dystrophy associated with misregulated splicing and altered gating of Ca(V)1.1 calcium channel. Hum Mol Genet, 21 (6): 1312-24. [PMID:22140091]
51. Toppin PJ, Chandy TT, Ghanekar A, Kraeva N, Beattie WS, Riazi S. (2010) A report of fulminant malignant hyperthermia in a patient with a novel mutation of the CACNA1S gene. Can J Anaesth, 57 (7): 689-93. [PMID:20431982]
52. Tuluc P, Molenda N, Schlick B, Obermair GJ, Flucher BE, Jurkat-Rott K. (2009) A CaV1.1 Ca2+ channel splice variant with high conductance and voltage-sensitivity alters EC coupling in developing skeletal muscle. Biophys J, 96 (1): 35-44. [PMID:19134469]
53. Walsh KB, Bryant SH, Schwartz A. (1986) Effect of calcium antagonist drugs on calcium currents in mammalian skeletal muscle fibers. J Pharmacol Exp Ther, 236 (2): 403-7. [PMID:2418195]
54. Wu F, Mi W, Hernández-Ochoa EO, Burns DK, Fu Y, Gray HF, Struyk AF, Schneider MF, Cannon SC. (2012) A calcium channel mutant mouse model of hypokalemic periodic paralysis. J Clin Invest, 122 (12): 4580-91. [PMID:23187123]
55. Wu J, Yan Z, Li Z, Qian X, Lu S, Dong M, Zhou Q, Yan N. (2016) Structure of the voltage-gated calcium channel Ca(v)1.1 at 3.6 Å resolution. Nature, 537 (7619): 191-196. [PMID:27580036]
56. Zhao Y, Huang G, Wu J, Wu Q, Gao S, Yan Z, Lei J, Yan N. (2019) Molecular Basis for Ligand Modulation of a Mammalian Voltage-Gated Ca2+ Channel. Cell, 177 (6): 1495-1506.e12. [PMID:31150622]