
GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Overview
The synthesis of cellular isoprenoids is an essential process, utilizing two alternative pathways (that have distinct evolutionary origins and are separated phylogenetically) to form the common intermediate metabolites isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). Most eukaryotes utilize the mevalonate (MVA) pathway (see our Lanosterol biosynthesis pathway page for more details), but phyla such as Plasmodium, that have acquired a plastid organelle, generate isoprenoids using the non-mevalonate or methylerythritol phosphate (MEP) pathway [2]. The complete pathway for P. falciparum is available at www.wikipathways.org and is shown below. Enzymes of the MEP pathway represent promising therapeutic targets for antimalarial drug development because the pathway is present in the Plasmodium parasite but not in the human host [1].
In the malaria parasite, the products of the MEP pathway are metabolized further by the bifunctional farnesyl/geranylgeranyl diphosphate synthase (PfFPPS/GGPPS) enzyme. Farnesyl diphosphate synthase (FPPS) catalyzes the coupling of IPP and DMAPP and is a key metabolic branch point in isoprenoid synthesis [3].
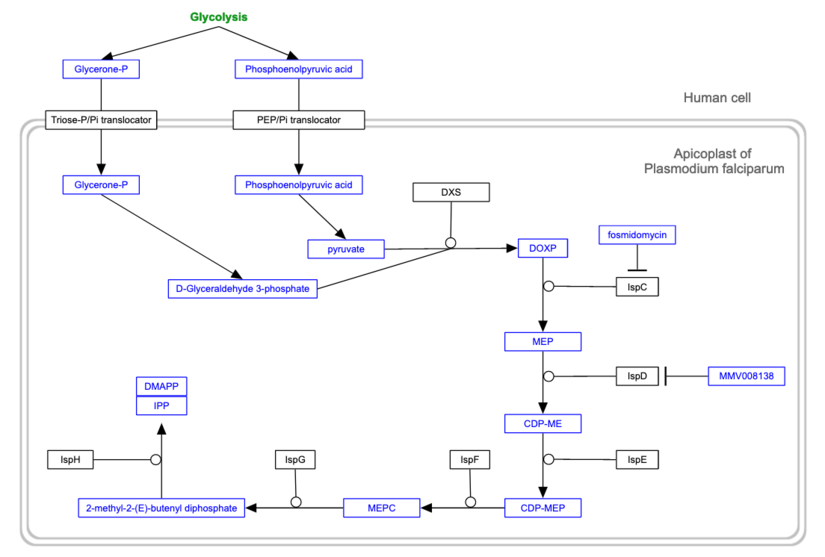
The P. falciparum MEP pathway (reproduced from Wikipathways).
Targets
|
PfDXR (Plasmodium falciparum 1-deoxy-D-xylulose 5-phosphate reductoisomerase)
C
Show summary »
More detailed page |
|
PfIspD (Plasmodium falciparum 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase)
C
Show summary »
More detailed page |
|
PfFPPS/GGPPS (Plasmodium falciparum bifunctional farnesyl/geranylgeranyl diphosphate synthase)
Show summary »
More detailed page |
How to cite this family page
Database page citation:
Isoprenoid biosynthesis enzymes . Accessed on 10/02/2026. IUPHAR/BPS Guide to PHARMACOLOGY, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=1055.
Concise Guide to PHARMACOLOGY citation:
Alexander SPH, Kelly E, Mathie AA, Peters JA, Veale EL, Armstrong JF, Buneman OP, Faccenda E, Harding SD, Spedding M, Cidlowski JA, Fabbro D, Davenport AP, Striessnig J, Davies JA et al. (2023) The Concise Guide to PHARMACOLOGY 2023/24: Introduction and Other Protein Targets. Br J Pharmacol. 180 Suppl 2:S1-22.








