Synonyms: C 4.6,S1-cyclo[N-(3-sulfanylpropanoyl)-D-tryptophyl-L-isoleucyl-L-alloisoleucyl-L-asparaginyl-L-2-aminobutanoyl-N-methyl-L-ornithinol] | FE 200 440 [ 2] | FE-200440
Comment: Barusiban (FE 200440) is an oxytocin receptor antagonist [ 1-2, 5]. It was developed by Ferring Pharmaceuticals as a tocolytic agent, to counteract oxytocin-induced myometrial contractions [ 4]. Chemically it is a synthetic cyclic hexapeptide containing five unnatural amino acids and only one D-amino acid.
|
|
2D Structure
|
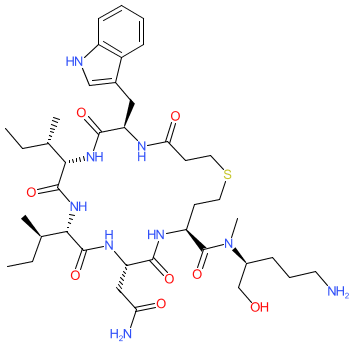
|
SMILES / InChI / InChIKey
|
|
|
Canonical SMILES
|
NCCC[C@H](N(C(=O)[C@@H]1CCSCCC(=O)N[C@H](Cc2c[nH]c3c2cccc3)C(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@H](C(=O)N1)CC(=O)N)[C@@H](CC)C)[C@H](CC)C)C)CO
|
|
Isomeric SMILES
|
NCCC[C@H](N(C(=O)[C@@H]1CCSCCC(=O)N[C@H](Cc2c[nH]c3c2cccc3)C(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@H](C(=O)N1)CC(=O)N)[C@@H](CC)C)[C@H](CC)C)C)CO
|
|
InChI
|
InChI=1S/C40H63N9O8S/c1-6-23(3)34-38(55)46-31(20-32(42)51)36(53)45-29(40(57)49(5)26(22-50)11-10-16-41)14-17-58-18-15-33(52)44-30(19-25-21-43-28-13-9-8-12-27(25)28)37(54)47-35(24(4)7-2)39(56)48-34/h8-9,12-13,21,23-24,26,29-31,34-35,43,50H,6-7,10-11,14-20,22,41H2,1-5H3,(H2,42,51)(H,44,52)(H,45,53)(H,46,55)(H,47,54)(H,48,56)/t23-,24+,26+,29+,30-,31+,34+,35+/m1/s1
|
|
InChI Key
|
UGNGRKKDUVKQDF-IHOMMZCZSA-N
|
Generated using the Chemistry Development Kit (CDK) (Willighagen EL et al. Journal of Cheminformatics vol. 9:33. 2017, doi:10.1186/s13321-017-0220-4; https://cdk.github.io/)
|
|


