
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Target not currently curated in GtoImmuPdb
Target id: 2062
Nomenclature: mitogen-activated protein kinase kinase 1
Abbreviated Name: MEK1
Family: STE7 family
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 393 | 15q22.31 | MAP2K1 | mitogen-activated protein kinase kinase 1 | |
| Mouse | - | 393 | 9 34.55 cM | Map2k1 | mitogen-activated protein kinase kinase 1 | |
| Rat | - | 393 | 8q24 | Map2k1 | mitogen activated protein kinase kinase 1 | |
Previous and Unofficial Names  |
| dual specificity mitogen-activated protein kinase kinase 1 | ERK activator kinase 1 | MAP kinase kinase 1 | MAPKK1 | MKK1 | PRKMK1 |
Database Links  |
|
| Alphafold | Q02750 (Hs), P31938 (Mm), Q01986 (Rn) |
| BRENDA | 2.7.12.2 |
| ChEMBL Target | CHEMBL3587 (Hs), CHEMBL5860 (Mm) |
| DrugBank Target | Q02750 (Hs) |
| Ensembl Gene | ENSG00000169032 (Hs), ENSMUSG00000004936 (Mm), ENSRNOG00000010176 (Rn) |
| Entrez Gene | 5604 (Hs), 26395 (Mm), 170851 (Rn) |
| Human Protein Atlas | ENSG00000169032 (Hs) |
| KEGG Enzyme | 2.7.12.2 |
| KEGG Gene | hsa:5604 (Hs), mmu:26395 (Mm), rno:170851 (Rn) |
| OMIM | 176872 (Hs) |
| Orphanet | ORPHA123135 (Hs) |
| Pharos | Q02750 (Hs) |
| RefSeq Nucleotide | NM_002755 (Hs), NM_008927 (Mm), NM_031643 (Rn) |
| RefSeq Protein | NP_002746 (Hs), NP_032953 (Mm), NP_113831 (Rn) |
| SynPHARM |
85068 (in complex with CH4987655) 83118 (in complex with cobimetinib) 80833 (in complex with PD 0325901) 80845 (in complex with refametinib) 80157 (in complex with selumetinib) 85069 (in complex with TAK-733) |
| UniProtKB | Q02750 (Hs), P31938 (Mm), Q01986 (Rn) |
| Wikipedia | MAP2K1 (Hs) |
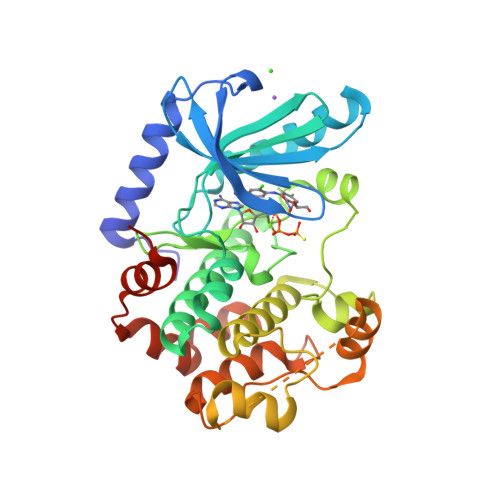
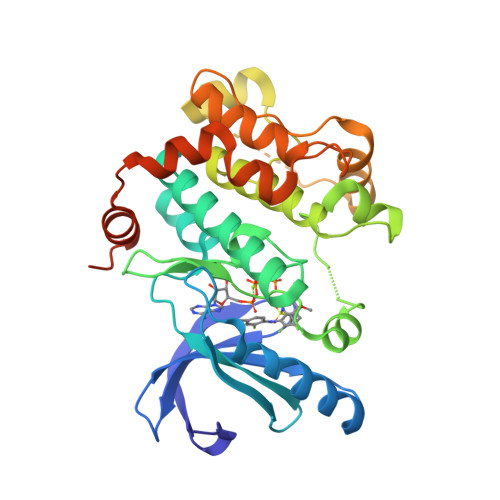
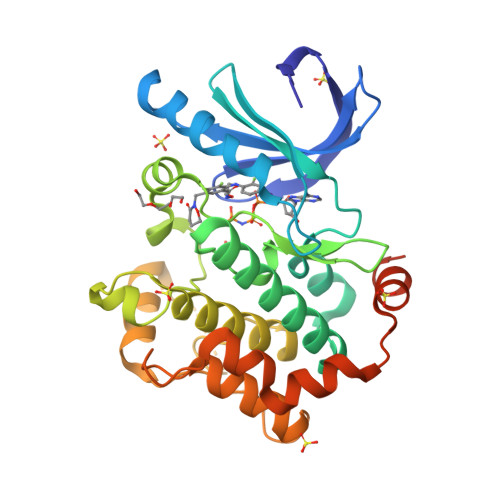
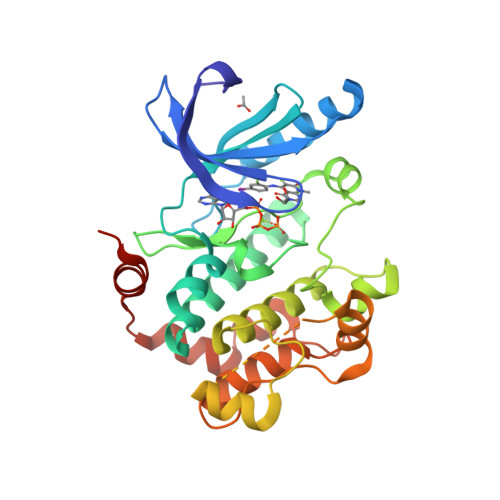
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inhibitor Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Trametinib inhibits both MAP2K1 (MEK1) and MAP2K2 (MEK2). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulators | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 4,25 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: MEK1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: 1,8 |
 
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: MEK1/MEK1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||
|
||||||||||||
1. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377]
2. Aoki T, Hyohdoh I, Furuichi N, Ozawa S, Watanabe F, Matsushita M, Sakaitani M, Morikami K, Takanashi K, Harada N et al.. (2014) Optimizing the Physicochemical Properties of Raf/MEK Inhibitors by Nitrogen Scanning. ACS Med Chem Lett, 5 (4): 309-14. [PMID:24900832]
3. Davies SP, Reddy H, Caivano M, Cohen P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J, 351 (Pt 1): 95-105. [PMID:10998351]
4. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
5. Dong Q, Dougan DR, Gong X, Halkowycz P, Jin B, Kanouni T, O'Connell SM, Scorah N, Shi L, Wallace MB et al.. (2011) Discovery of TAK-733, a potent and selective MEK allosteric site inhibitor for the treatment of cancer. Bioorg Med Chem Lett, 21 (5): 1315-9. [PMID:21310613]
6. Fedorov O, Marsden B, Pogacic V, Rellos P, Müller S, Bullock AN, Schwaller J, Sundström M, Knapp S. (2007) A systematic interaction map of validated kinase inhibitors with Ser/Thr kinases. Proc Natl Acad Sci USA, 104 (51): 20523-8. [PMID:18077363]
7. Fischmann TO, Smith CK, Mayhood TW, Myers JE, Reichert P, Mannarino A, Carr D, Zhu H, Wong J, Yang RS et al.. (2009) Crystal structures of MEK1 binary and ternary complexes with nucleotides and inhibitors. Biochemistry, 48 (12): 2661-74. [PMID:19161339]
8. Gao Y, Davies SP, Augustin M, Woodward A, Patel UA, Kovelman R, Harvey KJ. (2013) A broad activity screen in support of a chemogenomic map for kinase signalling research and drug discovery. Biochem J, 451 (2): 313-28. [PMID:23398362]
9. Gilmartin AG, Bleam MR, Groy A, Moss KG, Minthorn EA, Kulkarni SG, Rominger CM, Erskine S, Fisher KE, Yang J et al.. (2011) GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res, 17 (5): 989-1000. [PMID:21245089]
10. Goto M, Chow J, Muramoto K, Chiba K, Yamamoto S, Fujita M, Obaishi H, Tai K, Mizui Y, Tanaka I et al.. (2009) E6201 [(3S,4R,5Z,8S,9S,11E)-14-(ethylamino)-8, 9,16-trihydroxy-3,4-dimethyl-3,4,9,19-tetrahydro-1H-2-benzoxacyclotetradecine-1,7(8H)-dione], a novel kinase inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK)-1 and MEK kinase-1: in vitro characterization of its anti-inflammatory and antihyperproliferative activities. J Pharmacol Exp Ther, 331 (2): 485-95. [PMID:19684251]
11. Hartung IV, Hitchcock M, Pühler F, Neuhaus R, Scholz A, Hammer S, Petersen K, Siemeister G, Brittain D, Hillig RC. (2013) Optimization of allosteric MEK inhibitors. Part 1: Venturing into underexplored SAR territories. Bioorg Med Chem Lett, 23 (8): 2384-90. [PMID:23474388]
12. Hatzivassiliou G, Haling JR, Chen H, Song K, Price S, Heald R, Hewitt JF, Zak M, Peck A, Orr C et al.. (2013) Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature, 501 (7466): 232-6. [PMID:23934108]
13. Huynh H, Soo KC, Chow PK, Tran E. (2007) Targeted inhibition of the extracellular signal-regulated kinase kinase pathway with AZD6244 (ARRY-142886) in the treatment of hepatocellular carcinoma. Mol Cancer Ther, 6 (1): 138-46. [PMID:17237274]
14. Isshiki Y, Kohchi Y, Iikura H, Matsubara Y, Asoh K, Murata T, Kohchi M, Mizuguchi E, Tsujii S, Hattori K et al.. (2011) Design and synthesis of novel allosteric MEK inhibitor CH4987655 as an orally available anticancer agent. Bioorg Med Chem Lett, 21 (6): 1795-801. [PMID:21316218]
15. Iverson C, Larson G, Lai C, Yeh LT, Dadson C, Weingarten P, Appleby T, Vo T, Maderna A, Vernier JM et al.. (2009) RDEA119/BAY 869766: a potent, selective, allosteric inhibitor of MEK1/2 for the treatment of cancer. Cancer Res, 69 (17): 6839-47. [PMID:19706763]
16. Kincaid J, Duncton M. (2021) Pyrrolopyridine-aniline compounds for treatment of dermal disorders. Patent number: US11161845B2. Assignee: Nflection Therapeutics Inc. Priority date: 18/05/2018. Publication date: 02/11/2021.
17. Pheneger J, Wallace, E, Marlow, A Hurley B, Lyssikatos J, Bendele AM, Lee PA. (2006) Characterization of ARRY-438162, a Potent MEK Inhibitor in Combination with Methotrexate or Ibuprofen in In Vivo Models of Arthritis.[abstract]. American College of Rheumatology 2006 Annual Scientific Meeting,: Abstract 794.
18. Poddutoori R, Aardalen K, Aithal K, Barahagar SS, Belliappa C, Bock M, Chelur S, Gerken A, Gopinath S, Gruenenfelder B et al.. (2022) Discovery of MAP855, an Efficacious and Selective MEK1/2 Inhibitor with an ATP-Competitive Mode of Action. J Med Chem, 65 (5): 4350-4366. [PMID:35195996]
19. Rice KD, Aay N, Anand NK, Blazey CM, Bowles OJ, Bussenius J, Costanzo S, Curtis JK, Defina SC, Dubenko L et al.. (2012) Novel Carboxamide-Based Allosteric MEK Inhibitors: Discovery and Optimization Efforts toward XL518 (GDC-0973). ACS Med Chem Lett, 3 (5): 416-21. [PMID:24900486]
20. Sini P, Gürtler U, Zahn SK, Baumann C, Rudolph D, Baumgartinger R, Strauss E, Haslinger C, Tontsch-Grunt U, Waizenegger IC et al.. (2016) Pharmacological Profile of BI 847325, an Orally Bioavailable, ATP-Competitive Inhibitor of MEK and Aurora Kinases. Mol Cancer Ther, 15 (10): 2388-2398. [PMID:27496137]
21. Tecle H, Shao J, Li Y, Kothe M, Kazmirski S, Penzotti J, Ding YH, Ohren J, Moshinsky D, Coli R et al.. (2009) Beyond the MEK-pocket: can current MEK kinase inhibitors be utilized to synthesize novel type III NCKIs? Does the MEK-pocket exist in kinases other than MEK?. Bioorg Med Chem Lett, 19 (1): 226-9. [PMID:19019675]
22. Vollmer S, Cunoosamy D, Lv H, Feng H, Li X, Nan Z, Yang W, Perry MWD. (2020) Design, Synthesis, and Biological Evaluation of MEK PROTACs. J Med Chem, 63 (1): 157-162. DOI: 10.1021/acs.jmedchem.9b00810 [PMID:31804822]
23. Wei J, Hu J, Wang L, Xie L, Jin MS, Chen X, Liu J, Jin J. (2019) Discovery of a First-in-Class Mitogen-Activated Protein Kinase Kinase 1/2 Degrader. J Med Chem, 62 (23): 10897-10911. DOI: 10.1021/acs.jmedchem.9b01528 [PMID:31730343]
24. Wityak J, Hobbs FW, Gardner DS, Santella 3rd JB, Petraitis JJ, Sun JH, Favata MF, Daulerio AJ, Horiuchi KY, Copeland RA et al.. (2004) Beyond U0126. Dianion chemistry leading to the rapid synthesis of a series of potent MEK inhibitors. Bioorg Med Chem Lett, 14 (6): 1483-6. [PMID:15006386]
25. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
26. Yamaguchi T, Kakefuda R, Tajima N, Sowa Y, Sakai T. (2011) Antitumor activities of JTP-74057 (GSK1120212), a novel MEK1/2 inhibitor, on colorectal cancer cell lines in vitro and in vivo. Int J Oncol, 39 (1): 23-31. [PMID:21523318]
27. Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, Parry J, Smith D, Brandhuber BJ, Gross S et al.. (2007) Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res, 13 (5): 1576-83. [PMID:17332304]
STE7 family: mitogen-activated protein kinase kinase 1. Last modified on 11/08/2023. Accessed on 14/12/2025. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2062.