
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 400 | 6q25.2 | OPRM1 | opioid receptor mu 1 | 184 |
| Mouse | 7 | 398 | 10 1.85 cM | Oprm1 | opioid receptor, mu 1 | 114 |
| Rat | 7 | 398 | 1q11 | Oprm1 | opioid receptor, mu 1 | 27,47,161,183,194 |
Previous and Unofficial Names  |
| Mu receptor | MOP | OP3 | MOPr | opioid receptor, mu 1 | opioid receptor |
Database Links  |
|
| Specialist databases | |
| GPCRdb | oprm_human (Hs), oprm_mouse (Mm), oprm_rat (Rn) |
| Other databases | |
| Alphafold | P35372 (Hs), P42866 (Mm), P33535 (Rn) |
| ChEMBL Target | CHEMBL233 (Hs), CHEMBL2858 (Mm), CHEMBL270 (Rn) |
| DrugBank Target | P35372 (Hs) |
| Ensembl Gene | ENSG00000112038 (Hs), ENSMUSG00000000766 (Mm), ENSRNOG00000018191 (Rn) |
| Entrez Gene | 4988 (Hs), 18390 (Mm), 25601 (Rn) |
| Human Protein Atlas | ENSG00000112038 (Hs) |
| KEGG Gene | hsa:4988 (Hs), mmu:18390 (Mm), rno:25601 (Rn) |
| OMIM | 600018 (Hs) |
| Pharos | P35372 (Hs) |
| RefSeq Nucleotide | NM_000914 (Hs), NM_001039652 (Mm), NM_013071 (Rn) |
| RefSeq Protein | NP_000905 (Hs), NP_001034741 (Mm), NP_037203 (Rn) |
| SynPHARM | 3941 (in complex with β-FNA) |
| UniProtKB | P35372 (Hs), P42866 (Mm), P33535 (Rn) |
| Wikipedia | OPRM1 (Hs) |
Selected 3D Structures  |
|||||||||||||
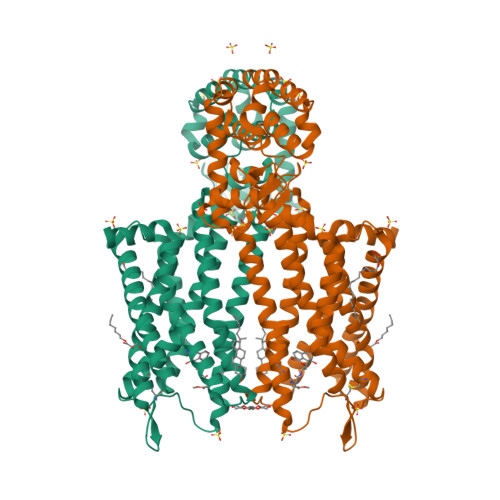
|
|
||||||||||||
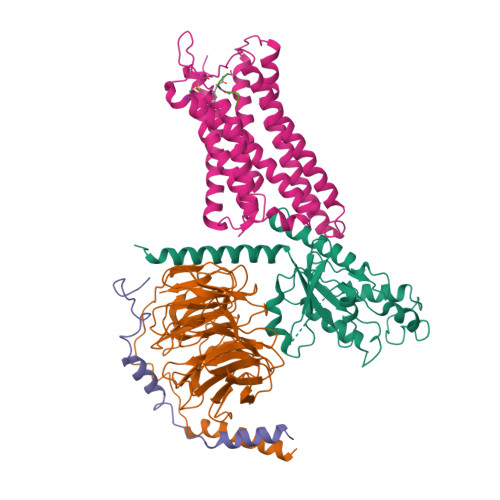
|
|
||||||||||||
Natural/Endogenous Ligands  |
| dynorphin A-(1-13) {Sp: Human, Mouse, Rat} |
| dynorphin A {Sp: Human, Mouse, Rat} |
| dynorphin A-(1-8) {Sp: Human, Mouse, Rat} |
| dynorphin B {Sp: Human, Mouse, Rat} |
| endomorphin-1 {Sp: Human} |
| endomorphin-2 {Sp: Human} |
| β-endorphin {Sp: Human} , β-endorphin {Sp: Mouse} , β-endorphin {Sp: Rat} |
| [Leu]enkephalin {Sp: Human, Mouse, Rat} |
| [Met]enkephalin {Sp: Human, Mouse, Rat} |
| Comments: β-Endorphin is the highest potency endogenous ligand |
| Potential endogenous agonists |
| endomorphin-1, endomorphin-2 |
| Principal endogenous agonists (Human) |
| β-endorphin (POMC, P01189), [Met]enkephalin (PENK, P01210), [Leu]enkephalin (PENK, P01210) |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific agonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discrimination of full or partial agonism is very dependent on the level of receptor expression and on the assay used to monitor agonist effects. Many agents may behave as full agonists or potent partial agonists in cell lines expressing cloned receptors in high concentration, but in other environments they may show only weak agonist activity. The identification of agonist activity in the table is largely based on the ability to stimulate GTPγ35S binding in cell lines expressing cloned human μ receptors. Agents giving 85% or greater stimulation than that given by DAMGO have been characterized as Full Agonists [166]. It is still unclear whether endomorphins are endogenous. Morphine occurs endogenously [139]. We have tagged the μ receptor as the primary drug target for hydrocodone based on this drug having the highest affinity at this receptor compared to the κ and δ receptors [128]. Similarly, we have tagged the μ receptor as the primary target of hydromorphone [185]. Methadone is selective for the μ receptor: comparable IC50s at the κ and δ receptors are 512 and 1090nM respectively [140]. [11C]carfentanil (PubChem CID 449698) binds the human μ receptor with a Ki of 0.07 nM, in a [3H]DAMGO displacement assay [67]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| β-FNA is an electrophilic affinity label. The pKi reflects both the reversible and irreversible binding components. CTOP is a good somatostatin receptor (sst receptor) agonist in addition to having antagonist activity at μ opioid receptors; it should never be used in studies of μ receptor function in situations where sst receptors may be involved. CTAP does not activate sst receptors [33]. The μ receptor is tagged as the primary target for the drug levallorphan, since the drug is mainly used for its antagonistic actions as an antidote to opioid overdose. Note that this drug also acts as a partial agonist at the κ receptor. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulators | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific allosteric modulator tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulator Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMS-986123 and BMS-986124 are Silent Allosteric Modulators (SAMs). These compounds neither potentiate nor inhibit the actions of an orthosteric agonist, however they block the actions of the Positive Allosteric Modulators (PAMs). A number of proteins such as G protein-coupled receptor kinases, β-arrestins and G proteins clearly regulate μ opioid receptor affinities and function. In addition sodium and guanyl nucleotides can modify the functional μ receptor complex and G protein interaction. Also, μ receptors are reported to form heterodimers with other receptors of the OP family or with non-opioid G protein-coupled receptors. Heterodimerisation may alter μ receptor function and/or trafficking [51,56,134]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other Binding Ligands | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
| Gi/Go family |
Adenylyl cyclase stimulation Adenylyl cyclase inhibition Phospholipase C stimulation Potassium channel Calcium channel Phospholipase A2 stimulation Phospholipase D stimulation Other - See Comments |
Comments:
The following systems have also been reported to be activated following Gi/Go activation via the μ receptor:
|
|
| References: 6,17,20,23,36,48,68,83,116,119,125,130,137-138,144,170,190 | |
Secondary Transduction Mechanisms  |
|
| Transducer | Effector/Response |
| Gq/G11 family | Phospholipase C stimulation |
| Comments: G16 couples to the μ opioid receptor and activates PLC. | |
| References: 70,93 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
| Tissue Distribution Comments | ||||||||
| μ opioid receptors are widely distributed with dense labelling throughout the fore, mid and hindbrain regions in the CNS. Quantitatively, the μ receptor is the most highly expressed of all the opioid receptors. Although the early studies used non-selective ligands such as [3H]dihydromorphine, characterisation of the distribution of the μ opioid receptor has been aided by the availability of [3H]DAMGO, a highly selective opioid agonist that has been the ligand of choice for labelling μ opioid receptors for over 20 years. Immunohistochemistry has largely confirmed receptor autoradiography. For a review of μ opioid receptor expression in the rat see [105]. |
||||||||
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Biologically Significant Variants 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
| Biologically Significant Variant Comments | ||||||||||
| There are many additional polymorphisms of the μ receptor which are either without function or their functional significance is presently unknown. |
1. Abbadie C, Pan YX, Pasternak GW. (2000) Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol, 419 (2): 244-56. [PMID:10723002]
2. Abbadie C, Pan YX, Pasternak GW. (2004) Immunohistochemical study of the expression of exon11-containing mu opioid receptor variants in mouse brain. Neuroscience, 127 (2): 419-30. [PMID:15262332]
3. Abbadie C, Pasternak GW. (2001) Differential in vivo internalization of MOR-1 and MOR-1C by morphine. Neuroreport, 12 (14): 3069-72. [PMID:11568638]
4. Arora S, Keenan SM, Peng Y, Welsh W, Zhang Q. (2006) Opioid receptor subtype-selective agents. Patent number: WO2006124687 A1. Assignee: Arora S, Keenan SM, Peng Y, Welsh W, Zhang Q.. Priority date: 12/05/2005. Publication date: 23/11/2006.
5. Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. (1995) Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci, 15 (5 Pt 1): 3328-41. [PMID:7751913]
6. Avidor-Reiss T, Nevo I, Saya D, Bayewitch M, Vogel Z. (1997) Opiate-induced adenylyl cyclase superactivation is isozyme-specific. J Biol Chem, 272 (8): 5040-7. [PMID:9030567]
7. Bagnol D, Mansour A, Akil H, Watson SJ. (1997) Cellular localization and distribution of the cloned mu and kappa opioid receptors in rat gastrointestinal tract. Neuroscience, 81 (2): 579-91. [PMID:9300443]
8. Baptista-Hon DT, Smith M, Singleton S, Antonides LH, Nic Daeid N, McKenzie C, Hales TG. (2020) Activation of μ-opioid receptors by MT-45 (1-cyclohexyl-4-(1,2-diphenylethyl)piperazine) and its fluorinated derivatives. Br J Pharmacol, 177 (15): 3436-3448. [PMID:32246840]
9. Becker A, Grecksch G, Brödemann R, Kraus J, Peters B, Schroeder H, Thiemann W, Loh HH, Höllt V. (2000) Morphine self-administration in mu-opioid receptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol, 361 (6): 584-9. [PMID:10882032]
10. Becker A, Grecksch G, Kraus J, Loh HH, Schroeder H, Höllt V. (2002) Rewarding effects of ethanol and cocaine in mu opioid receptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol, 365 (4): 296-302. [PMID:11919654]
11. Befort K, Filliol D, Decaillot FM, Gaveriaux-Ruff C, Hoehe MR, Kieffer BL. (2001) A single nucleotide polymorphic mutation in the human mu-opioid receptor severely impairs receptor signaling. J Biol Chem, 276 (5): 3130-7. [PMID:11067846]
12. Belcheva MM, Szùcs M, Wang D, Sadee W, Coscia CJ. (2001) mu-Opioid receptor-mediated ERK activation involves calmodulin-dependent epidermal growth factor receptor transactivation. J Biol Chem, 276 (36): 33847-53. [PMID:11457825]
13. Berrendero F, Kieffer BL, Maldonado R. (2002) Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J Neurosci, 22 (24): 10935-40. [PMID:12486188]
14. Besse D, Lombard MC, Besson JM. (1991) Autoradiographic distribution of mu, delta and kappa opioid binding sites in the superficial dorsal horn, over the rostrocaudal axis of the rat spinal cord. Brain Res, 548 (1-2): 287-91. [PMID:1651143]
15. Bolan EA, Pan YX, Pasternak GW. (2004) Functional analysis of MOR-1 splice variants of the mouse mu opioid receptor gene Oprm. Synapse, 51 (1): 11-8. [PMID:14579421]
16. Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM et al.. (1998) Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA, 95 (16): 9608-13. [PMID:9689128]
17. Bourinet E, Soong TW, Stea A, Snutch TP. (1996) Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci USA, 93 (4): 1486-91. [PMID:8643659]
18. Breslin HJ, Diamond CJ, Kavash RW, Cai C, Dyatkin AB, Miskowski TA, Zhang SP, Wade PR, Hornby PJ, He W. (2012) Identification of a dual δ OR antagonist/μ OR agonist as a potential therapeutic for diarrhea-predominant Irritable Bowel Syndrome (IBS-d). Bioorg Med Chem Lett, 22 (14): 4869-72. [PMID:22695132]
19. Burford NT, Clark MJ, Wehrman TS, Gerritz SW, Banks M, O'Connell J, Traynor JR, Alt A. (2013) Discovery of positive allosteric modulators and silent allosteric modulators of the μ-opioid receptor. Proc Natl Acad Sci USA, 110 (26): 10830-5. [PMID:23754417]
20. Carter BD, Medzihradsky F. (1993) Go mediates the coupling of the mu opioid receptor to adenylyl cyclase in cloned neural cells and brain. Proc Natl Acad Sci USA, 90 (9): 4062-6. [PMID:8097884]
21. Chakraborty S, DiBerto JF, Faouzi A, Bernhard SM, Gutridge AM, Ramsey S, Zhou Y, Provasi D, Nuthikattu N, Jilakara R et al.. (2021) A Novel Mitragynine Analog with Low-Efficacy Mu Opioid Receptor Agonism Displays Antinociception with Attenuated Adverse Effects. J Med Chem, 64 (18): 13873-13892. [PMID:34505767]
22. Chambers DR, Sulima A, Luo D, Prisinzano TE, Goldberg A, Xie B, Shi L, Paronis CA, Bergman J, Nassehi N et al.. (2022) A Journey through Diastereomeric Space: The Design, Synthesis, In Vitro and In Vivo Pharmacological Activity, and Molecular Modeling of Novel Potent Diastereomeric MOR Agonists and Antagonists. Molecules, 27 (19). DOI: 10.3390/molecules27196455 [PMID:36234992]
23. Chan JS, Chiu TT, Wong YH. (1995) Activation of type II adenylyl cyclase by the cloned mu-opioid receptor: coupling to multiple G proteins. J Neurochem, 65 (6): 2682-9. [PMID:7595566]
24. Chang KJ, Rigdon GC, Howard JL, McNutt RW. (1993) A novel, potent and selective nonpeptidic delta opioid receptor agonist BW373U86. J Pharmacol Exp Ther, 267 (2): 852-7. [PMID:8246159]
25. Chang KJ, Wei ET, Killian A, Chang JK. (1983) Potent morphiceptin analogs: structure activity relationships and morphine-like activities. J Pharmacol Exp Ther, 227 (2): 403-8. [PMID:6313901]
26. Chefer VI, Kieffer BL, Shippenberg TS. (2003) Basal and morphine-evoked dopaminergic neurotransmission in the nucleus accumbens of MOR- and DOR-knockout mice. Eur J Neurosci, 18 (7): 1915-22. [PMID:14622224]
27. Chen Y, Mestek A, Liu J, Hurley JA, Yu L. (1993) Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol, 44 (1): 8-12. [PMID:8393525]
28. Chen Z, Davies E, Miller WS, Shan S, Valenzano KJ, Kyle DJ. (2004) Design and synthesis of 4-phenyl piperidine compounds targeting the mu receptor. Bioorg Med Chem Lett, 14 (21): 5275-9. [PMID:15454210]
29. Cheney BV, Szmuszkovicz J, Lahti RA, Zichi DA. (1985) Factors affecting binding of trans-N-[2-(methylamino)cyclohexyl]benzamides at the primary morphine receptor. J Med Chem, 28 (12): 1853-64. [PMID:2999404]
30. Cheng JT, Liu IM, Hsu CF. (2003) Rapid induction of insulin resistance in opioid mu-receptor knock-out mice. Neurosci Lett, 339 (2): 139-42. [PMID:12614914]
31. Cheng PY, Liu-Chen LY, Pickel VM. (1997) Dual ultrastructural immunocytochemical labeling of mu and delta opioid receptors in the superficial layers of the rat cervical spinal cord. Brain Res, 778 (2): 367-80. [PMID:9459554]
32. Cheng PY, Moriwaki A, Wang JB, Uhl GR, Pickel VM. (1996) Ultrastructural localization of mu-opioid receptors in the superficial layers of the rat cervical spinal cord: extrasynaptic localization and proximity to Leu5-enkephalin. Brain Res, 731 (1-2): 141-54. [PMID:8883864]
33. Chieng B, Connor M, Christie MJ. (1996) The mu-opioid receptor antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) [but not D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP)] produces a nonopioid receptor-mediated increase in K+ conductance of rat locus ceruleus neurons. Mol Pharmacol, 50 (3): 650-5. [PMID:8794906]
34. Chuang TK, Killam Jr KF, Chuang LF, Kung HF, Sheng WS, Chao CC, Yu L, Chuang RY. (1995) Mu opioid receptor gene expression in immune cells. Biochem Biophys Res Commun, 216 (3): 922-30. [PMID:7488213]
35. Cometta-Morini C, Maguire PA, Loew GH. (1992) Molecular determinants of mu receptor recognition for the fentanyl class of compounds. Mol Pharmacol, 41 (1): 185-96. [PMID:1310142]
36. Connor M, Schuller A, Pintar JE, Christie MJ. (1999) Mu-opioid receptor modulation of calcium channel current in periaqueductal grey neurons from C57B16/J mice and mutant mice lacking MOR-1. Br J Pharmacol, 126 (7): 1553-8. [PMID:10323586]
37. Contarino A, Picetti R, Matthes HW, Koob GF, Kieffer BL, Gold LH. (2002) Lack of reward and locomotor stimulation induced by heroin in mu-opioid receptor-deficient mice. Eur J Pharmacol, 446 (1-3): 103-9. [PMID:12098591]
38. Dekan Z, Sianati S, Yousuf A, Sutcliffe KJ, Gillis A, Mallet C, Singh P, Jin AH, Wang AM, Mohammadi SA et al.. (2019) A tetrapeptide class of biased analgesics from an Australian fungus targets the µ-opioid receptor. Proc Natl Acad Sci USA, 116 (44): 22353-22358. [PMID:31611414]
39. Delfs JM, Kong H, Mestek A, Chen Y, Yu L, Reisine T, Chesselet MF. (1994) Expression of mu opioid receptor mRNA in rat brain: an in situ hybridization study at the single cell level. J Comp Neurol, 345 (1): 46-68. [PMID:8089277]
40. Dietis N, McDonald J, Molinari S, Calo G, Guerrini R, Rowbotham DJ, Lambert DG. (2012) Pharmacological characterization of the bifunctional opioid ligand H-Dmt-Tic-Gly-NH-Bzl (UFP-505). Br J Anaesth, 108 (2): 262-70. [PMID:22194444]
41. Dietis N, Niwa H, Tose R, McDonald J, Ruggieri V, Filaferro M, Vitale G, Micheli L, Ghelardini C, Salvadori S et al.. (2018) In vitro and in vivo characterization of the bifunctional μ and δ opioid receptor ligand UFP-505. Br J Pharmacol, 175 (14): 2881-2896. [PMID:29524334]
42. Ding YQ, Kaneko T, Nomura S, Mizuno N. (1996) Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol, 367 (3): 375-402. [PMID:8698899]
43. Ding YQ, Nomura S, Kaneko T, Mizuno N. (1995) Co-localization of mu-opioid receptor-like and substance P-like immunoreactivities in axon terminals within the superficial layers of the medullary and spinal dorsal horns of the rat. Neurosci Lett, 198 (1): 45-8. [PMID:8570093]
44. Ding YQ, Nomura S, Kaneko T, Mizuno N. (1995) Presynaptic localization of mu-opioid receptor-like immunoreactivity in retinal axon terminals within the terminal nuclei of the accessory optic tract: a light and electron microscope study in the rat. Neurosci Lett, 199 (2): 139-42. [PMID:8584243]
45. Fan XL, Zhang JS, Zhang XQ, Ma L. (2003) Chronic morphine treatment and withdrawal induce up-regulation of c-Jun N-terminal kinase 3 gene expression in rat brain. Neuroscience, 122 (4): 997-1002. [PMID:14643766]
46. Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M et al.. (2000) Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet, 25 (2): 195-200. [PMID:10835636]
47. Fukuda K, Kato S, Mori K, Nishi M, Takeshima H. (1993) Primary structures and expression from cDNAs of rat opioid receptor delta- and mu-subtypes. FEBS Lett, 327: 311-314. [PMID:8394245]
48. Fukuda K, Kato S, Morikawa H, Shoda T, Mori K. (1996) Functional coupling of the delta-, mu-, and kappa-opioid receptors to mitogen-activated protein kinase and arachidonate release in Chinese hamster ovary cells. J Neurochem, 67 (3): 1309-16. [PMID:8752140]
49. Fulton BS, Knapp BI, Bidlack JM, Neumeyer JL. (2008) Synthesis and pharmacological evaluation of hydrophobic esters and ethers of butorphanol at opioid receptors. Bioorg Med Chem Lett, 18 (16): 4474-6. [PMID:18674902]
50. Gavériaux-Ruff C, Matthes HW, Peluso J, Kieffer BL. (1998) Abolition of morphine-immunosuppression in mice lacking the mu-opioid receptor gene. Proc Natl Acad Sci USA, 95 (11): 6326-30. [PMID:9600964]
51. George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, O'Dowd BF. (2000) Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem, 275 (34): 26128-35. [PMID:10842167]
52. George SR, Zastawny RL, Briones-Urbina R, Cheng R, Nguyen T, Heiber M, Kouvelas A, Chan AS, O'Dowd BF. (1994) Distinct distributions of mu, delta and kappa opioid receptor mRNA in rat brain. Biochem Biophys Res Commun, 205 (2): 1438-44. [PMID:7802680]
53. Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. (2002) Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci, 22 (3): 1146-54. [PMID:11826143]
54. Glatfelter GC, Vandeputte MM, Chen L, Walther D, Tsai MM, Shi L, Stove CP, Baumann MH. (2023) Alkoxy chain length governs the potency of 2-benzylbenzimidazole 'nitazene' opioids associated with human overdose. Psychopharmacology (Berl), 240 (12): 2573-2584. [PMID:37658878]
55. Goldstein A, Naidu A. (1989) Multiple opioid receptors: ligand selectivity profiles and binding site signatures. Mol Pharmacol, 36 (2): 265-72. [PMID:2549383]
56. Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. (2000) Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci, 20 (22): RC110. [PMID:11069979]
57. Gong J, Strong JA, Zhang S, Yue X, DeHaven RN, Daubert JD, Cassel JA, Yu G, Mansson E, Yu L. (1998) Endomorphins fully activate a cloned human mu opioid receptor. FEBS Lett, 439 (1-2): 152-6. [PMID:9849897]
58. Guerrero M, Urbano M, Kim EK, Gamo AM, Riley S, Abgaryan L, Leaf N, Van Orden LJ, Brown SJ, Xie JY et al.. (2019) Design and Synthesis of a Novel and Selective Kappa Opioid Receptor (KOR) Antagonist (BTRX-335140). J Med Chem, 62 (4): 1761-1780. [PMID:30707578]
59. Gulya K, Pelton JT, Hruby VJ, Yamamura HI. (1986) Cyclic somatostatin octapeptide analogues with high affinity and selectivity toward mu opioid receptors. Life Sci, 38 (24): 2221-9. [PMID:2872570]
60. Gutman ES, Bow E, Li F, Sulima A, Kaska S, Crowley R, Prisinzano TE, Lee YS, Hassan SA, Imler GH et al.. (2020) G-Protein biased opioid agonists: 3-hydroxy-N-phenethyl-5-phenylmorphans with three-carbon chain substituents at C9. RSC Med Chem, 11 (8): 896-904. [PMID:33479684]
61. Hall FS, Goeb M, Li XF, Sora I, Uhl GR. (2004) mu-Opioid receptor knockout mice display reduced cocaine conditioned place preference but enhanced sensitization of cocaine-induced locomotion. Brain Res Mol Brain Res, 121 (1-2): 123-30. [PMID:14969743]
62. Hall FS, Sora I, Uhl GR. (2001) Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology (Berl.), 154 (1): 43-9. [PMID:11292005]
63. Handa BK, Land AC, Lord JA, Morgan BA, Rance MJ, Smith CF. (1981) Analogues of beta-LPH61-64 possessing selective agonist activity at mu-opiate receptors. Eur J Pharmacol, 70 (4): 531-40. [PMID:6263640]
64. Handler CM, Geller EB, Adler MW. (1992) Effect of mu-, kappa-, and delta-selective opioid agonists on thermoregulation in the rat. Pharmacol Biochem Behav, 43 (4): 1209-16. [PMID:1361992]
65. Hanessian S, Parthasarathy S, Mauduit M, Payza K. (2003) The power of visual imagery in drug design. Isopavines as a new class of morphinomimetics and their human opioid receptor binding activity. J Med Chem, 46 (1): 34-48. [PMID:12502358]
66. Hawkins KN, Morelli M, Gulya K, Chang KJ, Yamamura HI. (1987) Autoradiographic localization of [3H] [MePhe3,D-Pro4]morphiceptin ([3H]PL017) to mu opioid receptors in rat brain. Eur J Pharmacol, 133 (3): 351-2. [PMID:3030778]
67. Henriksen G, Platzer S, Marton J, Hauser A, Berthele A, Schwaiger M, Marinelli L, Lavecchia A, Novellino E, Wester HJ. (2005) Syntheses, biological evaluation, and molecular modeling of 18F-labeled 4-anilidopiperidines as mu-opioid receptor imaging agents. J Med Chem, 48 (24): 7720-32. [PMID:16302812]
68. Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. (1996) Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature, 380 (6571): 258-62. [PMID:8637576]
69. Hiebel AC, Lee YS, Bilsky E, Giuvelis D, Deschamps JR, Parrish DA, Aceto MD, May EL, Harris LS, Coop A et al.. (2007) Probes for narcotic receptor mediated phenomena. 34. Synthesis and structure-activity relationships of a potent mu-agonist delta-antagonist and an exceedingly potent antinociceptive in the enantiomeric C9-substituted 5-(3-hydroxyphenyl)-N-phenylethylmorphan series. J Med Chem, 50 (16): 3765-76. [PMID:17625813]
70. Ho MK, New DC, Wong YH. (2002) Co-expressions of different opioid receptor types differentially modulate their signaling via G(16). Neurosignals, 11 (2): 115-22. [PMID:12077485]
71. Husbands SM, Lewis JW. (1995) Morphinan cyclic imines and pyrrolidines containing a constrained phenyl group: high affinity opioid agonists. Bioorg Med Chem Lett, 5: 2969–2974.
72. Hutcheson DM, Matthes HW, Valjent E, Sánchez-Blázquez P, Rodríguez-Díaz M, Garzón J, Kieffer BL, Maldonado R. (2001) Lack of dependence and rewarding effects of deltorphin II in mu-opioid receptor-deficient mice. Eur J Neurosci, 13 (1): 153-61. [PMID:11135013]
73. Inagaki M, Kume M, Tamura Y, Hara S, Goto Y, Haga N, Hasegawa T, Nakamura T, Koike K, Oonishi S et al.. (2019) Discovery of naldemedine: A potent and orally available opioid receptor antagonist for treatment of opioid-induced adverse effects. Bioorg Med Chem Lett, 29 (1): 73-77. [PMID:30446313]
74. Jamot L, Matthes HW, Simonin F, Kieffer BL, Roder JC. (2003) Differential involvement of the mu and kappa opioid receptors in spatial learning. Genes Brain Behav, 2 (2): 80-92. [PMID:12884965]
75. Jang CG, Lee SY, Yoo JH, Yan JJ, Song DK, Loh HH, Ho IK. (2003) Impaired water maze learning performance in mu-opioid receptor knockout mice. Brain Res Mol Brain Res, 117 (1): 68-72. [PMID:14499482]
76. Jongkamonwiwat N, Phansuwan-Pujito P, Sarapoke P, Chetsawang B, Casalotti SO, Forge A, Dodson H, Govitrapong P. (2003) The presence of opioid receptors in rat inner ear. Hear Res, 181 (1-2): 85-93. [PMID:12855366]
77. Kam AY, Chan AS, Wong YH. (2004) Phosphatidylinositol-3 kinase is distinctively required for mu-, but not kappa-opioid receptor-induced activation of c-Jun N-terminal kinase. J Neurochem, 89 (2): 391-402. [PMID:15056283]
78. Kaneko T, Minami M, Satoh M, Mizuno N. (1995) Immunocytochemical localization of mu-opioid receptor in the rat caudate-putamen. Neurosci Lett, 184 (3): 149-52. [PMID:7715834]
79. Kelly E, Mundell SJ, Sava A, Roth AL, Felici A, Maltby K, Nathan PJ, Bullmore ET, Henderson G. (2015) The opioid receptor pharmacology of GSK1521498 compared to other ligands with differential effects on compulsive reward-related behaviours. Psychopharmacology (Berl.), 232 (1): 305-14. [PMID:24973897]
80. Kennedy NM, Schmid CL, Ross NC, Lovell KM, Yue Z, Chen YT, Cameron MD, Bohn LM, Bannister TD. (2018) Optimization of a Series of Mu Opioid Receptor (MOR) Agonists with High G Protein Signaling Bias. J Med Chem, 61 (19): 8895-8907. [PMID:30199635]
81. Khroyan TV, Polgar WE, Cami-Kobeci G, Husbands SM, Zaveri NT, Toll L. (2011) The first universal opioid ligand, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028): characterization of the in vitro profile and in vivo behavioral effects in mouse models of acute pain and cocaine-induced reward. J Pharmacol Exp Ther, 336 (3): 952-61. [PMID:21177476]
82. Kitchen I, Slowe SJ, Matthes HW, Kieffer B. (1997) Quantitative autoradiographic mapping of mu-, delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain Res, 778 (1): 73-88. [PMID:9462879]
83. Koch T, Brandenburg LO, Schulz S, Liang Y, Klein J, Hollt V. (2003) ADP-ribosylation factor-dependent phospholipase D2 activation is required for agonist-induced mu-opioid receptor endocytosis. J Biol Chem, 278: 9979-9985. [PMID:12519790]
84. Koch T, Kroslak T, Averbeck M, Mayer P, Schröder H, Raulf E, Höllt V. (2000) Allelic variation S268P of the human mu-opioid receptor affects both desensitization and G protein coupling. Mol Pharmacol, 58 (2): 328-34. [PMID:10908300]
85. Koch T, Schulz S, Pfeiffer M, Klutzny M, Schröder H, Kahl E, Höllt V. (2001) C-terminal splice variants of the mouse mu-opioid receptor differ in morphine-induced internalization and receptor resensitization. J Biol Chem, 276 (33): 31408-14. [PMID:11359768]
86. Koehl A, Hu H, Maeda S, Zhang Y, Qu Q, Paggi JM, Latorraca NR, Hilger D, Dawson R, Matile H et al.. (2018) Structure of the µ-opioid receptor-Gi protein complex. Nature, 558 (7711): 547-552. [PMID:29899455]
87. Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA. (2005) Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol Rev, 57 (1): 1-26. [PMID:15734726]
88. Kumar V, Guo D, Marella M, Cassel JA, Dehaven RN, Daubert JD, Mansson E. (2008) Use of receptor chimeras to identify small molecules with high affinity for the dynorphin A binding domain of the kappa opioid receptor. Bioorg Med Chem Lett, 18 (12): 3667-71. [PMID:18487043]
89. Kvam TM, Baar C, Rakvåg TT, Kaasa S, Krokan HE, Skorpen F. (2004) Genetic analysis of the murine mu opioid receptor: increased complexity of Oprm gene splicing. J Mol Med, 82 (4): 250-5. [PMID:14991152]
90. Le Bourdonnec B, Barker WM, Belanger S, Wiant DD, Conway-James NC, Cassel JA, O'Neill TJ, Little PJ, DeHaven RN, DeHaven-Hudkins DL et al.. (2008) Novel trans-3,4-dimethyl-4-(3-hydroxyphenyl)piperidines as mu opioid receptor antagonists with improved opioid receptor selectivity profiles. Bioorg Med Chem Lett, 18 (6): 2006-12. [PMID:18313920]
91. Le Bourdonnec B, Windh RT, Ajello CW, Leister LK, Gu M, Chu GH, Tuthill PA, Barker WM, Koblish M, Wiant DD et al.. (2008) Potent, orally bioavailable delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4'-piperidine]-4-yl)benzamide (ADL5859). J Med Chem, 51 (19): 5893-6. [PMID:18788723]
92. Le Bourdonnec B, Windh RT, Leister LK, Zhou QJ, Ajello CW, Gu M, Chu GH, Tuthill PA, Barker WM, Koblish M et al.. (2009) Spirocyclic delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-3-hydroxy-4-(spiro[chromene-2,4'-piperidine]-4-yl) benzamide (ADL5747). J Med Chem, 52 (18): 5685-702. [PMID:19694468]
93. Lee JW, Joshi S, Chan JS, Wong YH. (1998) Differential coupling of mu-, delta-, and kappa-opioid receptors to G alpha16-mediated stimulation of phospholipase C. J Neurochem, 70 (5): 2203-11. [PMID:9572309]
94. Lei W, Vekariya RH, Ananthan S, Streicher JM. (2020) A Novel Mu-Delta Opioid Agonist Demonstrates Enhanced Efficacy With Reduced Tolerance and Dependence in Mouse Neuropathic Pain Models. J Pain, 21 (1-2): 146-160. [PMID:31201990]
95. Li JL, Ding YQ, Li YQ, Li JS, Nomura S, Kaneko T, Mizuno N. (1998) Immunocytochemical localization of mu-opioid receptor in primary afferent neurons containing substance P or calcitonin gene-related peptide. A light and electron microscope study in the rat. Brain Res, 794 (2): 347-52. [PMID:9622672]
96. Li LY, Chang KJ. (1996) The stimulatory effect of opioids on mitogen-activated protein kinase in Chinese hamster ovary cells transfected to express mu-opioid receptors. Mol Pharmacol, 50 (3): 599-602. [PMID:8794899]
97. Linz K, Christoph T, Tzschentke TM, Koch T, Schiene K, Gautrois M, Schröder W, Kögel BY, Beier H, Englberger W et al.. (2014) Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther, 349 (3): 535-48. [PMID:24713140]
98. Livingston KE, Stanczyk MA, Burford NT, Alt A, Canals M, Traynor JR. (2018) Pharmacologic Evidence for a Putative Conserved Allosteric Site on Opioid Receptors. Mol Pharmacol, 93 (2): 157-167. [PMID:29233847]
99. Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. (1998) mu Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res, 54 (2): 321-6. [PMID:9555078]
100. Lötsch J, Geisslinger G. (2005) Are mu-opioid receptor polymorphisms important for clinical opioid therapy?. Trends Mol Med, 11 (2): 82-9. [PMID:15694871]
101. Majumdar S, Burgman M, Haselton N, Grinnell S, Ocampo J, Pasternak AR, Pasternak GW. (2011) Generation of novel radiolabeled opiates through site-selective iodination. Bioorg Med Chem Lett, 21 (13): 4001-4. [PMID:21621410]
102. Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. (2012) Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature, 485 (7398): 321-6. [PMID:22437502]
103. Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H et al.. (2016) Structure-based discovery of opioid analgesics with reduced side effects. Nature, 537 (7619): 185-190. [PMID:27533032]
104. Mangoura D. (1997) mu-Opioids activate tyrosine kinase focal adhesion kinase and regulate cortical cytoskeleton proteins cortactin and vinculin in chick embryonic neurons. J Neurosci Res, 50 (3): 391-401. [PMID:9364324]
105. Mansour A, Fox CA, Akil H, Watson SJ. (1995) Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci, 18: 22-29. [PMID:7535487]
106. Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. (1994) Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol, 350: 412-438. [PMID:7884049]
107. Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ. (1994) mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain Res, 643 (1-2): 245-65. [PMID:8032920]
108. Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. (1987) Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci, 7 (8): 2445-64. [PMID:3039080]
109. Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P et al.. (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature, 383 (6603): 819-23. [PMID:8893006]
110. Matthies H, Schroeder H, Becker A, Loh H, Höllt V, Krug M. (2000) Lack of expression of long-term potentiation in the dentate gyrus but not in the CA1 region of the hippocampus of mu-opioid receptor-deficient mice. Neuropharmacology, 39 (6): 952-60. [PMID:10727705]
111. Mayer P, Höllt V. (2005) Genetic disposition to addictive disorders--current knowledge and future perspectives. Curr Opin Pharmacol, 5 (1): 4-8. [PMID:15661619]
112. Mayer P, Schulzeck S, Kraus J, Zimprich A, Höllt V. (1996) Promoter region and alternatively spliced exons of the rat mu-opioid receptor gene. J Neurochem, 66 (6): 2272-8. [PMID:8632148]
113. McCarthy L, Szabo I, Nitsche JF, Pintar JE, Rogers TJ. (2001) Expression of functional mu-opioid receptors during T cell development. J Neuroimmunol, 114 (1-2): 173-80. [PMID:11240029]
114. Min BH, Augustin LB, Felsheim RF, Fuchs JA, Loh HH. (1994) Genomic structure analysis of promoter sequence of a mouse mu opioid receptor gene. Proc Natl Acad Sci USA, 91 (19): 9081-5. [PMID:8090773]
115. Minami M, Onogi T, Toya T, Katao Y, Hosoi Y, Maekawa K, Katsumata S, Yabuuchi K, Satoh M. (1994) Molecular cloning and in situ hybridization histochemistry for rat mu-opioid receptor. Neurosci Res, 18: 315-322. [PMID:8190373]
116. Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. (2003) Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences. Proc Natl Acad Sci USA, 100 (1): 271-6. [PMID:12496346]
117. Miyazaki T, Choi IY, Rubas W, Anand NK, Ali C, Evans J, Gursahani H, Hennessy M, Kim G, McWeeney D et al.. (2017) NKTR-181: A Novel Mu-Opioid Analgesic with Inherently Low Abuse Potential. J Pharmacol Exp Ther, 363 (1): 104-113. [PMID:28778859]
118. Moles A, Kieffer BL, D'Amato FR. (2004) Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science, 304 (5679): 1983-6. [PMID:15218152]
119. Morikawa H, Fukuda K, Kato S, Mori K, Higashida H. (1995) Coupling of the cloned mu-opioid receptor with the omega-conotoxin-sensitive Ca2+ current in NG108-15 cells. J Neurochem, 65 (3): 1403-6. [PMID:7643119]
120. Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, Beaudet A. (2003) Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. J Neurosci, 23 (12): 4888-98. [PMID:12832511]
121. Morinville A, Cahill CM, Kieffer B, Collier B, Beaudet A. (2004) Mu-opioid receptor knockout prevents changes in delta-opioid receptor trafficking induced by chronic inflammatory pain. Pain, 109 (3): 266-73. [PMID:15157687]
122. Moriwaki A, Wang JB, Svingos A, van Bockstaele E, Cheng P, Pickel V, Uhl GR. (1996) mu Opiate receptor immunoreactivity in rat central nervous system. Neurochem Res, 21 (11): 1315-31. [PMID:8947922]
123. Mrkusich EM, Kivell BM, Miller JH, Day DJ. (2004) Abundant expression of mu and delta opioid receptor mRNA and protein in the cerebellum of the fetal, neonatal, and adult rat. Brain Res Dev Brain Res, 148: 213-222. [PMID:14766199]
124. Murray RB, Adler MW, Korczyn AD. (1983) The pupillary effects of opioids. Life Sci, 33: 495-509. [PMID:6136886]
125. Murthy KS, Makhlouf GM. (1996) Opioid mu, delta, and kappa receptor-induced activation of phospholipase C-beta 3 and inhibition of adenylyl cyclase is mediated by Gi2 and G(o) in smooth muscle. Mol Pharmacol, 50 (4): 870-7. [PMID:8863832]
126. Narita M, Imai S, Narita M, Kasukawa A, Yajima Y, Suzuki T. (2004) Increased level of neuronal phosphoinositide 3-kinase gamma by the activation of mu-opioid receptor in the mouse periaqueductal gray matter: further evidence for the implication in morphine-induced antinociception. Neuroscience, 124 (3): 515-21. [PMID:14980723]
127. Narita M, Imai S, Ozaki S, Suzuki M, Narita M, Suzuki T. (2003) Reduced expression of a novel mu-opioid receptor (MOR) subtype MOR-1B in CXBK mice: implications of MOR-1B in the expression of MOR-mediated responses. Eur J Neurosci, 18 (12): 3193-8. [PMID:14686893]
128. Neumeyer JL, Zhang B, Zhang T, Sromek AW, Knapp BI, Cohen DJ, Bidlack JM. (2012) Synthesis, binding affinity, and functional in vitro activity of 3-benzylaminomorphinan and 3-benzylaminomorphine ligands at opioid receptors. J Med Chem, 55 (8): 3878-90. [PMID:22439881]
129. Nikolaev VO, Boettcher C, Dees C, Bünemann M, Lohse MJ, Zenk MH. (2007) Live cell monitoring of mu-opioid receptor-mediated G-protein activation reveals strong biological activity of close morphine biosynthetic precursors. J Biol Chem, 282 (37): 27126-27132. [PMID:17616524]
130. North RA, Williams JT, Surprenant A, Christie MJ. (1987) Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci USA, 84 (15): 5487-91. [PMID:2440052]
131. Pak Y, Kouvelas A, Scheideler MA, Rasmussen J, O'Dowd BF, George SR. (1996) Agonist-induced functional desensitization of the mu-opioid receptor is mediated by loss of membrane receptors rather than uncoupling from G protein. Mol Pharmacol, 50 (5): 1214-22. [PMID:8913353]
132. Pan YX, Xu J, Mahurter L, Bolan E, Xu M, Pasternak GW. (2001) Generation of the mu opioid receptor (MOR-1) protein by three new splice variants of the Oprm gene. Proc Natl Acad Sci USA, 98 (24): 14084-9. [PMID:11717463]
133. Patkar KA, Yan X, Murray TF, Aldrich JV. (2005) [Nalpha-benzylTyr1,cyclo(D-Asp5,Dap8)]- dynorphin A-(1-11)NH2 cyclized in the "address" domain is a novel kappa-opioid receptor antagonist. J Med Chem, 48 (14): 4500-3. [PMID:15999987]
134. Pfeiffer M, Kirscht S, Stumm R, Koch T, Wu D, Laugsch M, Schröder H, Höllt V, Schulz S. (2003) Heterodimerization of substance P and mu-opioid receptors regulates receptor trafficking and resensitization. J Biol Chem, 278 (51): 51630-7. [PMID:14532289]
135. Phansuwan-Pujito P, Saleema L, Mukda S, Tongjaroenbuangam W, Jutapakdeegul N, Casalotti SO, Forge A, Dodson H, Govitrapong P. (2003) The opioid receptors in inner ear of different stages of postnatal rats. Hear Res, 184: 1-10. [PMID:14553898]
136. Philippe D, Dubuquoy L, Groux H, Brun V, Chuoï-Mariot MT, Gaveriaux-Ruff C, Colombel JF, Kieffer BL, Desreumaux P. (2003) Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest, 111 (9): 1329-38. [PMID:12727924]
137. Piros ET, Prather PL, Law PY, Evans CJ, Hales TG. (1996) Voltage-dependent inhibition of Ca2+ channels in GH3 cells by cloned mu- and delta-opioid receptors. Mol Pharmacol, 50 (4): 947-56. [PMID:8863841]
138. Piros ET, Prather PL, Loh HH, Law PY, Evans CJ, Hales TG. (1995) Ca2+ channel and adenylyl cyclase modulation by cloned mu-opioid receptors in GH3 cells. Mol Pharmacol, 47 (5): 1041-9. [PMID:7746271]
139. Poeaknapo C, Schmidt J, Brandsch M, Dräger B, Zenk MH. (2004) Endogenous formation of morphine in human cells. Proc Natl Acad Sci USA, 101 (39): 14091-6. [PMID:15383669]
140. Poulain R, Horvath D, Bonnet B, Eckhoff C, Chapelain B, Bodinier MC, Déprez B. (2001) From hit to lead. Combining two complementary methods for focused library design. Application to mu opiate ligands. J Med Chem, 44 (21): 3378-90. [PMID:11585443]
141. Prchalová E, Hin N, Thomas AG, Veeravalli V, Ng J, Alt J, Rais R, Rojas C, Li Z, Hihara H et al.. (2019) Discovery of Benzamidine- and 1-Aminoisoquinoline-Based Human MAS-Related G-Protein-Coupled Receptor X1 (MRGPRX1) Agonists. J Med Chem, 62 (18): 8631-8641. [PMID:31498617]
142. Qu Q, Huang W, Aydin D, Paggi JM, Seven AB, Wang H, Chakraborty S, Che T, DiBerto JF, Robertson MJ et al.. (2023) Insights into distinct signaling profiles of the µOR activated by diverse agonists. Nat Chem Biol, 19 (4): 423-430. [PMID:36411392]
143. Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. (1994) Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol, 45 (2): 330-4. [PMID:8114680]
144. Rhim H, Miller RJ. (1994) Opioid receptors modulate diverse types of calcium channels in the nucleus tractus solitarius of the rat. J Neurosci, 14 (12): 7608-15. [PMID:7996199]
145. Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, Forster BM, Wong CJ, Li X, Crile RS et al.. (2014) LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology, 77: 131-44. [PMID:24071566]
146. Roy S, Balasubramanian S, Sumandeep S, Charboneau R, Wang J, Melnyk D, Beilman GJ, Vatassery R, Barke RA. (2001) Morphine directs T cells toward T(H2) differentiation. Surgery, 130 (2): 304-9. [PMID:11490364]
147. Roy S, Barke RA, Loh HH. (1998) MU-opioid receptor-knockout mice: role of mu-opioid receptor in morphine mediated immune functions. Brain Res Mol Brain Res, 61 (1-2): 190-4. [PMID:9795212]
148. Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. (2004) The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci, 19 (8): 2239-48. [PMID:15090050]
149. Schnell SA, Wessendorf MW. (2004) Expression of MOR1C-like mu-opioid receptor mRNA in rats. J Comp Neurol, 473 (2): 213-32. [PMID:15101090]
150. Schulz S, Schreff M, Koch T, Zimprich A, Gramsch C, Elde R, Höllt V. (1998) Immunolocalization of two mu-opioid receptor isoforms (MOR1 and MOR1B) in the rat central nervous system. Neuroscience, 82 (2): 613-22. [PMID:9466465]
151. Sharif NA, Hughes J. (1989) Discrete mapping of brain Mu and delta opioid receptors using selective peptides: quantitative autoradiography, species differences and comparison with kappa receptors. Peptides, 10 (3): 499-522. [PMID:2550910]
152. Sim LJ, Selley DE, Childers SR. (1995) In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5'-[gamma-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA, 92 (16): 7242-6. [PMID:7638174]
153. Singhal P, Kapasi A, Reddy K, Franki N. (2001) Opiates promote T cell apoptosis through JNK and caspase pathway. Adv Exp Med Biol, 493: 127-135. [PMID:11727758]
154. Sora I, Funada M, Uhl GR. (1997) The mu-opioid receptor is necessary for [D-Pen2,D-Pen5]enkephalin-induced analgesia. Eur J Pharmacol, 324 (2-3): R1-2. [PMID:9145787]
155. Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. (1997) Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA, 94 (4): 1544-9. [PMID:9037090]
156. Spetea M, Berzetei-Gurske IP, Guerrieri E, Schmidhammer H. (2012) Discovery and pharmacological evaluation of a diphenethylamine derivative (HS665), a highly potent and selective κ opioid receptor agonist. J Med Chem, 55 (22): 10302-6. [PMID:23134120]
157. Ständer S, Gunzer M, Metze D, Luger T, Steinhoff M. (2002) Localization of mu-opioid receptor 1A on sensory nerve fibers in human skin. Regul Pept, 110 (1): 75-83. [PMID:12468112]
158. Svingos AL, Moriwaki A, Wang JB, Uhl GR, Pickel VM. (1996) Ultrastructural immunocytochemical localization of mu-opioid receptors in rat nucleus accumbens: extrasynaptic plasmalemmal distribution and association with Leu5-enkephalin. J Neurosci, 16 (13): 4162-73. [PMID:8753878]
159. Svingos AL, Moriwaki A, Wang JB, Uhl GR, Pickel VM. (1997) mu-Opioid receptors are localized to extrasynaptic plasma membranes of GABAergic neurons and their targets in the rat nucleus accumbens. J Neurosci, 17 (7): 2585-94. [PMID:9065518]
160. Tempel A, Zukin RS. (1987) Neuroanatomical patterns of the mu, delta, and kappa opioid receptors of rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci USA, 84 (12): 4308-12. [PMID:3035579]
161. Thompson RC, Mansour A, Akil H, Watson SJ. (1993) Cloning and pharmacological characterization of a rat mu opioid receptor. Neuron, 11 (5): 903-13. [PMID:8240812]
162. Tian M, Broxmeyer HE, Fan Y, Lai Z, Zhang S, Aronica S, Cooper S, Bigsby RM, Steinmetz R, Engle SJ, Mestek A, Pollock JD, Lehman MN, Jansen HT, Ying M, Stambrook PJ, Tischfield JA, Yu L. (1997) Altered hematopoiesis, behavior, and sexual function in mu opioid receptor-deficient mice. J Exp Med, 185: 1517-1522. [PMID:9126934]
163. Tien LT, Fan LW, Sogawa C, Ma T, Loh HH, Ho IK. (2004) Changes in acetylcholinesterase activity and muscarinic receptor bindings in mu-opioid receptor knockout mice. Brain Res Mol Brain Res, 126 (1): 38-44. [PMID:15207914]
164. Tien LT, Park Y, Fan LW, Ma T, Loh HH, Ho IK. (2003) Increased dopamine D2 receptor binding and enhanced apomorphine-induced locomotor activity in mu-opioid receptor knockout mice. Brain Res Bull, 61 (1): 109-15. [PMID:12788214]
165. Toll L. (1990) Mu-opioid receptor binding in intact SH-SY5Y neuroblastoma cells. Eur J Pharmacol, 176 (2): 213-7. [PMID:1968847]
166. Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O'Brien A, White A et al.. (1998) Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr, 178: 440-66. [PMID:9686407]
167. Toll L, Khroyan TV, Polgar WE, Jiang F, Olsen C, Zaveri NT. (2009) Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/mu-opioid receptor ligands: implications for therapeutic applications. J Pharmacol Exp Ther, 331 (3): 954-64. [PMID:19773529]
168. Trafton JA, Abbadie C, Marek K, Basbaum AI. (2000) Postsynaptic signaling via the [mu]-opioid receptor: responses of dorsal horn neurons to exogenous opioids and noxious stimulation. J Neurosci, 20 (23): 8578-84. [PMID:11102461]
169. Tzschentke TM, Christoph T, Kögel B, Schiene K, Hennies HH, Englberger W, Haurand M, Jahnel U, Cremers TI, Friderichs E et al.. (2007) (-)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel mu-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther, 323 (1): 265-76. [PMID:17656655]
170. Ueda H, Miyamae T, Fukushima N, Takeshima H, Fukuda K, Sasaki Y, Misu Y. (1995) Opioid mu- and kappa-receptor mediate phospholipase C activation through Gi1 in Xenopus oocytes. Brain Res Mol Brain Res, 32 (1): 166-70. [PMID:7494457]
171. Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S et al.. (2016) Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit β-Arrestin-2. J Med Chem, 59 (18): 8381-97. [PMID:27556704]
172. Van Bockstaele EJ, Colago EE, Cheng P, Moriwaki A, Uhl GR, Pickel VM. (1996) Ultrastructural evidence for prominent distribution of the mu-opioid receptor at extrasynaptic sites on noradrenergic dendrites in the rat nucleus locus coeruleus. J Neurosci, 16: 5037-5048. [PMID:8756434]
173. Van Bockstaele EJ, Colago EE, Moriwaki A, Uhl GR. (1996) Mu-opioid receptor is located on the plasma membrane of dendrites that receive asymmetric synapses from axon terminals containing leucine-enkephalin in the rat nucleus locus coeruleus. J Comp Neurol, 376 (1): 65-74. [PMID:8946284]
174. Vandeputte MM, Tsai MM, Chen L, Glatfelter GC, Walther D, Stove CP, Shi L, Baumann MH. (2023) Comparative neuropharmacology of structurally distinct non-fentanyl opioids that are appearing on recreational drug markets worldwide. Drug Alcohol Depend, 249: 109939. [PMID:37276825]
175. Varty GB, Lu SX, Morgan CA, Cohen-Williams ME, Hodgson RA, Smith-Torhan A, Zhang H, Fawzi AB, Graziano MP, Ho GD et al.. (2008) The anxiolytic-like effects of the novel, orally active nociceptin opioid receptor agonist 8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol (SCH 221510). J Pharmacol Exp Ther, 326 (2): 672-82. [PMID:18492950]
176. Vergura R, Balboni G, Spagnolo B, Gavioli E, Lambert DG, McDonald J, Trapella C, Lazarus LH, Regoli D, Guerrini R et al.. (2008) Anxiolytic- and antidepressant-like activities of H-Dmt-Tic-NH-CH(CH2-COOH)-Bid (UFP-512), a novel selective delta opioid receptor agonist. Peptides, 29 (1): 93-103. [PMID:18069089]
177. Volpe DA, McMahon Tobin GA, Mellon RD, Katki AG, Parker RJ, Colatsky T, Kropp TJ, Verbois SL. (2011) Uniform assessment and ranking of opioid μ receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol, 59 (3): 385-90. [PMID:21215785]
178. Vu LY, Luo D, Johnson K, Denehy ED, Songrady JC, Martin J, Trivedi R, Alsum AR, Shaykin JD, Chaudhary CL et al.. (2024) Searching for Synthetic Opioid Rescue Agents: Identification of a Potent Opioid Agonist with Reduced Respiratory Depression. J Med Chem, 67 (11): 9173-9193. [PMID:38810170]
179. Wang D, Tolbert LM, Carlson KW, Sadée W. (2000) Nuclear Ca2+/calmodulin translocation activated by mu-opioid (OP3) receptor. J Neurochem, 74 (4): 1418-25. [PMID:10737597]
180. Wang H, Moriwaki A, Wang JB, Uhl GR, Pickel VM. (1997) Ultrastructural immunocytochemical localization of mu-opioid receptors in dendritic targets of dopaminergic terminals in the rat caudate-putamen nucleus. Neuroscience, 81 (3): 757-71. [PMID:9316027]
181. Wang H, Pélaprat D, Roques BP, Vanhove A, Chi ZQ, Rostène W. (1991) [3H]ohmefentanyl preferentially binds to mu-opioid receptors but also labels sigma-sites in rat brain sections. Eur J Pharmacol, 193 (3): 341-50. [PMID:1647320]
182. Wang J, Barke RA, Charboneau R, Loh HH, Roy S. (2003) Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J Biol Chem, 278 (39): 37622-31. [PMID:12842891]
183. Wang JB, Imai Y, Eppler CM, Gregor P, Spivak CE, Uhl GR. (1993) mu opiate receptor: cDNA cloning and expression. Proc Natl Acad Sci USA, 90 (21): 10230-4. [PMID:8234282]
184. Wang JB, Johnson PS, Persico AM, Hawkins AL, Griffin CA, Uhl GR. (1994) Human mu opiate receptor. cDNA and genomic clones, pharmacologic characterization and chromosomal assignment. FEBS Lett, 338 (2): 217-22. [PMID:7905839]
185. Wentland MP, Lou R, Lu Q, Bu Y, Denhardt C, Jin J, Ganorkar R, VanAlstine MA, Guo C, Cohen DJ et al.. (2009) Syntheses of novel high affinity ligands for opioid receptors. Bioorg Med Chem Lett, 19 (8): 2289-94. [PMID:19282177]
186. Wentland MP, Lu Q, Lou R, Bu Y, Knapp BI, Bidlack JM. (2005) Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett, 15 (8): 2107-10. [PMID:15808478]
187. Xie CW, Morrisett RA, Lewis DV. (1992) Mu opioid receptor-mediated modulation of synaptic currents in dentate granule cells of rat hippocampus. J Neurophysiol, 68 (4): 1113-20. [PMID:1359026]
188. Yeadon M, Kitchen I. (1988) Comparative binding of mu and delta selective ligands in whole brain and pons/medulla homogenates from rat: affinity profiles of fentanyl derivatives. Neuropharmacology, 27 (4): 345-8. [PMID:2843777]
189. Yoo JH, Yang EM, Lee SY, Loh HH, Ho IK, Jang CG. (2003) Differential effects of morphine and cocaine on locomotor activity and sensitization in mu-opioid receptor knockout mice. Neurosci Lett, 344 (1): 37-40. [PMID:12781916]
190. Yoshimura M, Ikeda H, Tabakoff B. (1996) mu-Opioid receptors inhibit dopamine-stimulated activity of type V adenylyl cyclase but enhance dopamine-stimulated activity of type VII adenylyl cyclase. Mol Pharmacol, 50 (1): 43-51. [PMID:8700117]
191. Yu VC, Eiger S, Duan DS, Lameh J, Sadée W. (1990) Regulation of cyclic AMP by the mu-opioid receptor in human neuroblastoma SH-SY5Y cells. J Neurochem, 55 (4): 1390-6. [PMID:1697894]
192. Yuen JW, So IY, Kam AY, Wong YH. (2004) Regulation of STAT3 by mu-opioid receptors in human neuroblastoma SH-SY5Y cells. Neuroreport, 15 (9): 1431-5. [PMID:15194868]
193. Zadina JE, Hackler L, Ge LJ, Kastin AJ. (1997) A potent and selective endogenous agonist for the mu-opiate receptor. Nature, 386 (6624): 499-502. [PMID:9087409]
194. Zastawny RL, George SR, Nguyen T, Cheng R, Tsatsos J, Briones-Urbina R, O'Dowd BF. (1994) Cloning, characterization, and distribution of a mu-opioid receptor in rat brain. J Neurochem, 62 (6): 2099-105. [PMID:8189219]
195. Zaveri NT, Journigan VB, Polgar WE. (2015) Discovery of the first small-molecule opioid pan antagonist with nanomolar affinity at mu, delta, kappa, and nociceptin opioid receptors. ACS Chem Neurosci, 6 (4): 646-57. [PMID:25635572]
196. Zheng Y, Obeng S, Wang H, Jali AM, Peddibhotla B, Williams DA, Zou C, Stevens DL, Dewey WL, Akbarali HI et al.. (2019) Design, Synthesis, and Biological Evaluation of the Third Generation 17-Cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[(4'-pyridyl)carboxamido]morphinan (NAP) Derivatives as μ/κ Opioid Receptor Dual Selective Ligands. J Med Chem, 62 (2): 561-574. [PMID:30608693]
197. Zhu Y, Pintar JE. (1998) Expression of opioid receptors and ligands in pregnant mouse uterus and placenta. Biol Reprod, 59 (4): 925-32. [PMID:9746745]
198. Zimprich A, Simon T, Höllt V. (1995) Cloning and expression of an isoform of the rat mu opioid receptor (rMOR1B) which differs in agonist induced desensitization from rMOR1. FEBS Lett, 359 (2-3): 142-6. [PMID:7532594]