
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
 target has curated data in GtoImmuPdb
target has curated data in GtoImmuPdb
Target id: 1499
Nomenclature: mitogen-activated protein kinase 14
Abbreviated Name: p38α
Family: p38 subfamily
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 360 | 6p21.31 | MAPK14 | mitogen-activated protein kinase 14 | |
| Mouse | - | 360 | 17 14.85 cM | Mapk14 | mitogen-activated protein kinase 14 | |
| Rat | - | 360 | 20p12 | Mapk14 | mitogen activated protein kinase 14 | |
Database Links  |
|
| Alphafold | Q16539 (Hs), P47811 (Mm), P70618 (Rn) |
| BRENDA | 2.7.11.24 |
| ChEMBL Target | CHEMBL260 (Hs), CHEMBL2336 (Mm), CHEMBL4825 (Rn) |
| Ensembl Gene | ENSG00000112062 (Hs), ENSMUSG00000053436 (Mm), ENSRNOG00000000513 (Rn) |
| Entrez Gene | 1432 (Hs), 26416 (Mm), 81649 (Rn) |
| Human Protein Atlas | ENSG00000112062 (Hs) |
| KEGG Enzyme | 2.7.11.24 |
| KEGG Gene | hsa:1432 (Hs), mmu:26416 (Mm), rno:81649 (Rn) |
| OMIM | 600289 (Hs) |
| Pharos | Q16539 (Hs) |
| RefSeq Nucleotide | NM_001315 (Hs), NM_001168508 (Mm), NM_031020 (Rn) |
| RefSeq Protein | NP_001306 (Hs), NP_001161985 (Mm), NP_001161980 (Mm), NP_001161986 (Mm), NP_036081 (Mm), NP_112282 (Rn) |
| SynPHARM |
80146 (in complex with AZD6703) 80672 (in complex with BMS-582949) 85089 (in complex with pamapimod) 80641 (in complex with PH-797804) 78583 (in complex with SB203580) 80561 (in complex with SB220025) 83879 (in complex with TAK-715) 80639 (in complex with talmapimod) 81051 (in complex with VX-745) |
| UniProtKB | Q16539 (Hs), P47811 (Mm), P70618 (Rn) |
| Wikipedia | MAPK14 (Hs) |
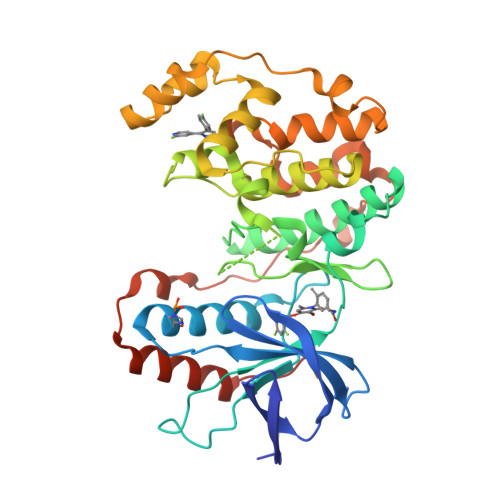
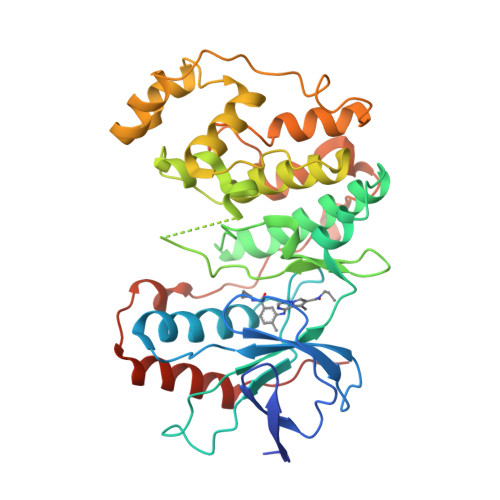
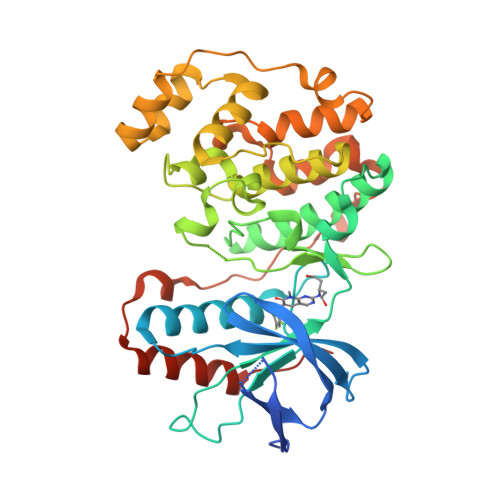
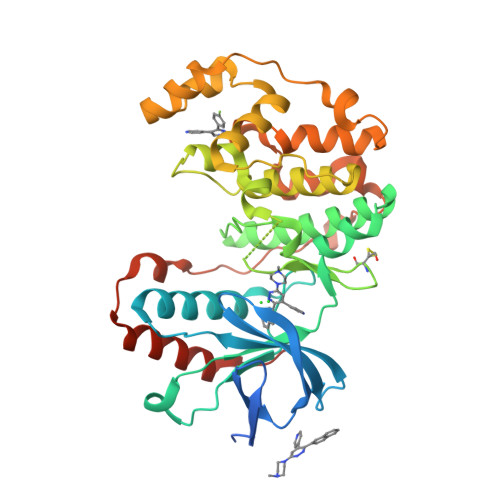
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 16,39 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: p38-alpha | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: 6,19 |
 
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: SAPK2a/P38a(MAPK14) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| p38 MAP kinases are ubiquitous, highly conserved enzymes which regulate the production of proinflammatory mediators (such as TNFα and IL-1) in response to inflammatory cytokines or environmental stress [23-24,27,33-34,38]. They are essential for normal immune and inflammatory responses, but are also involved in many other cellular processes such as regulating the cell cycle and cytoskeletal remodelling. Pharmacological inhibition of p38 MAP kinases reduces inflammatory cytokine synthesis, making these enzymes validated and extensively pursued drug targets for autoimmune and inflammatory diseases, including arthritis and other joint diseases, septic shock, myocardial injury and neuroinflammation. A number of pan-p38 MAP kinase inhibitors and isoform selective inhibitors have been evaluated in clinical trials. MAPK14 is ubiquitously expressed and its central role in pro-inflammatory signalling made it a popular drug target for chronic inflammatory diseases. However, MAPK14 inhibitors have failed to show clinical efficacy, most likely due to MAPK14's wide-spread tissue distrubution and/or off-target effects on other kinases. It is worth noting that knockout of Mapk14 in mice is embryonic lethal. |
1. Adams JL, Boehm JC, Gallagher TF, Kassis S, Webb EF, Hall R, Sorenson M, Garigipati R, Griswold DE, Lee JC. (2001) Pyrimidinylimidazole inhibitors of p38: cyclic N-1 imidazole substituents enhance p38 kinase inhibition and oral activity. Bioorg Med Chem Lett, 11 (21): 2867-70. [PMID:11597418]
2. Adams JL, Boehm JC, Hall R, Jin Q, Kasparec J, Silva DJ, Taggart JJ. Novel trisubstituted-8H-pyrido[2,3-d]pyrimidin-7-one compound for the treatment of CSBP/p38 kinase mediated diseases. Patent number: EP1333833. Assignee: GlaxoSmithKline LLC. Priority date: 23/10/2000. Publication date: 23/10/2001.
3. Aiguadé J, Balagué C, Carranco I, Caturla F, Domínguez M, Eastwood P, Esteve C, González J, Lumeras W, Orellana A et al.. (2012) Novel triazolopyridylbenzamides as potent and selective p38α inhibitors. Bioorg Med Chem Lett, 22 (10): 3431-6. [PMID:22521646]
4. Albrecht W, Unger A, Bauer SM, Laufer SA. (2017) Discovery of N-{4-[5-(4-Fluorophenyl)-3-methyl-2-methylsulfanyl-3H-imidazol-4-yl]-pyridin-2-yl}-acetamide (CBS-3595), a Dual p38α MAPK/PDE-4 Inhibitor with Activity against TNFα-Related Diseases. J Med Chem, 60 (13): 5290-5305. [PMID:28613871]
5. Alevy YG, Patel AC, Romero AG, Patel DA, Tucker J, Roswit WT, Miller CA, Heier RF, Byers DE, Brett TJ et al.. (2012) IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest, 122 (12): 4555-68. [PMID:23187130]
6. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377]
7. Arancio O, Watterson DM, Pelletier JC, Roy SM. (2014) Map kinase modulators and uses thereof. Patent number: WO2014145485A2. Assignee: The Trustees Of Columbia University In The City Of New York. Priority date: 15/03/2013. Publication date: 18/09/2014.
8. Armani E, Capaldi C, Bagnacani V, Saccani F, Aquino G, Puccini P, Facchinetti F, Martucci C, Moretto N, Villetti G et al.. (2022) Design, Synthesis, and Biological Characterization of Inhaled p38α/β MAPK Inhibitors for the Treatment of Lung Inflammatory Diseases. J Med Chem, 65 (10): 7170-7192. [PMID:35546685]
9. Aston NM, Bamborough P, Buckton JB, Edwards CD, Holmes DS, Jones KL, Patel VK, Smee PA, Somers DO, Vitulli G et al.. (2009) p38alpha mitogen-activated protein kinase inhibitors: optimization of a series of biphenylamides to give a molecule suitable for clinical progression. J Med Chem, 52 (20): 6257-69. [PMID:19772287]
10. Bachegowda L, Morrone K, Winski SL, Mantzaris I, Bartenstein M, Ramachandra N, Giricz O, Sukrithan V, Nwankwo G, Shahnaz S et al.. (2016) Pexmetinib: A Novel Dual Inhibitor of Tie2 and p38 MAPK with Efficacy in Preclinical Models of Myelodysplastic Syndromes and Acute Myeloid Leukemia. Cancer Res, 76 (16): 4841-4849. [PMID:27287719]
11. Badura L, Swanson T, Adamowicz W, Adams J, Cianfrogna J, Fisher K, Holland J, Kleiman R, Nelson F, Reynolds L et al.. (2007) An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J Pharmacol Exp Ther, 322 (2): 730-8. [PMID:17502429]
12. Bain J, McLauchlan H, Elliott M, Cohen P. (2003) The specificities of protein kinase inhibitors: an update. Biochem J, 371 (Pt 1): 199-204. [PMID:12534346]
13. Bhattacharjee D, Bakar J, Chitnis SP, Sausville EL, Ashtekar KD, Mendelson BE, Long K, Smith JC, Heppner DE, Sheltzer JM. (2023) Inhibition of a lower potency target drives the anticancer activity of a clinical p38 inhibitor. Cell Chem Biol, 30 (10): 1211-1222.e5. [PMID:37827156]
14. Brough S, Evans R, Luker TJ, Raubo P. (2008) Pyrazinone derivatives and their use in the treatment of lung diseases. Patent number: WO2009001132A1. Assignee: Astrazeneca. Priority date: 27/06/2007. Publication date: 31/12/2008.
15. Brown DS, Cumming JG, Bethel P, Finlayson J, Gerhardt S, Nash I, Pauptit RA, Pike KG, Reid A, Snelson W et al.. (2012) The discovery of N-cyclopropyl-4-methyl-3-[6-(4-methylpiperazin-1-yl)-4-oxoquinazolin-3(4H)-yl]benzamide (AZD6703), a clinical p38α MAP kinase inhibitor for the treatment of inflammatory diseases. Bioorg Med Chem Lett, 22 (12): 3879-83. [PMID:22608965]
16. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
17. Eyers PA, Craxton M, Morrice N, Cohen P, Goedert M. (1998) Conversion of SB 203580-insensitive MAP kinase family members to drug-sensitive forms by a single amino-acid substitution. Chem Biol, 5 (6): 321-8. [PMID:9653550]
18. Fryszman OM, Lang H, Lan J, Chang E, Fang Y. (2005) 5-membered heterocycle-based p38 kinase inhibitors. Patent number: WO2005009973. Assignee: Novartis Ag. Priority date: 26/06/2003. Publication date: 03/02/2005.
19. Gao Y, Davies SP, Augustin M, Woodward A, Patel UA, Kovelman R, Harvey KJ. (2013) A broad activity screen in support of a chemogenomic map for kinase signalling research and drug discovery. Biochem J, 451 (2): 313-28. [PMID:23398362]
20. Goldstein DM, Alfredson T, Bertrand J, Browner MF, Clifford K, Dalrymple SA, Dunn J, Freire-Moar J, Harris S, Labadie SS et al.. (2006) Discovery of S-[5-amino-1-(4-fluorophenyl)-1H-pyrazol-4-yl]-[3-(2,3-dihydroxypropoxy)phenyl]methanone (RO3201195), an orally bioavailable and highly selective inhibitor of p38 MAP kinase. J Med Chem, 49 (5): 1562-75. [PMID:16509574]
21. Goldstein DM, Kuglstatter A, Lou Y, Soth MJ. (2010) Selective p38alpha inhibitors clinically evaluated for the treatment of chronic inflammatory disorders. J Med Chem, 53 (6): 2345-53. [PMID:19950901]
22. Goldstein DM, Soth M, Gabriel T, Dewdney N, Kuglstatter A, Arzeno H, Chen J, Bingenheimer W, Dalrymple SA, Dunn J et al.. (2011) Discovery of 6-(2,4-difluorophenoxy)-2-[3-hydroxy-1-(2-hydroxyethyl)propylamino]-8-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (pamapimod) and 6-(2,4-difluorophenoxy)-8-methyl-2-(tetrahydro-2H-pyran-4-ylamino)pyrido[2,3-d]pyrimidin-7(8H)-one (R1487) as orally bioavailable and highly selective inhibitors of p38α mitogen-activated protein kinase. J Med Chem, 54 (7): 2255-65. [PMID:21375264]
23. Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. (1997) Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature, 386 (6622): 296-9. [PMID:9069290]
24. Han J, Lee JD, Bibbs L, Ulevitch RJ. (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science, 265 (5173): 808-11. [PMID:7914033]
25. Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT et al.. (2008) A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol, 26 (1): 127-32. [PMID:18183025]
26. Klüter S, Grütter C, Naqvi T, Rabiller M, Simard JR, Pawar V, Getlik M, Rauh D. (2010) Displacement assay for the detection of stabilizers of inactive kinase conformations. J Med Chem, 53 (1): 357-67. [PMID:19928858]
27. Lee JC, Kumar S, Griswold DE, Underwood DC, Votta BJ, Adams JL. (2000) Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology, 47 (2-3): 185-201. [PMID:10878289]
28. Liu C, Lin J, Wrobleski ST, Lin S, Hynes J, Wu H, Dyckman AJ, Li T, Wityak J, Gillooly KM et al.. (2010) Discovery of 4-(5-(cyclopropylcarbamoyl)-2-methylphenylamino)-5-methyl-N-propylpyrrolo[1,2-f][1,2,4]triazine-6-carboxamide (BMS-582949), a clinical p38α MAP kinase inhibitor for the treatment of inflammatory diseases. J Med Chem, 53 (18): 6629-39. [PMID:20804198]
29. Mader M, de Dios A, Shih C, Bonjouklian R, Li T, White W, López de Uralde B, Sánchez-Martinez C, del Prado M, Jaramillo C et al.. (2008) Imidazolyl benzimidazoles and imidazo[4,5-b]pyridines as potent p38alpha MAP kinase inhibitors with excellent in vivo antiinflammatory properties. Bioorg Med Chem Lett, 18 (1): 179-83. [PMID:18039577]
30. Millan DS, Bunnage ME, Burrows JL, Butcher KJ, Dodd PG, Evans TJ, Fairman DA, Hughes SJ, Kilty IC, Lemaitre A et al.. (2011) Design and synthesis of inhaled p38 inhibitors for the treatment of chronic obstructive pulmonary disease. J Med Chem, 54 (22): 7797-814. [PMID:21888439]
31. Miwatashi S, Arikawa Y, Kotani E, Miyamoto M, Naruo K, Kimura H, Tanaka T, Asahi S, Ohkawa S. (2005) Novel inhibitor of p38 MAP kinase as an anti-TNF-alpha drug: discovery of N-[4-[2-ethyl-4-(3-methylphenyl)-1,3-thiazol-5-yl]-2-pyridyl]benzamide (TAK-715) as a potent and orally active anti-rheumatoid arthritis agent. J Med Chem, 48 (19): 5966-79. [PMID:16162000]
32. Moffett K, Konteatis Z, Nguyen D, Shetty R, Ludington J, Fujimoto T, Lee KJ, Chai X, Namboodiri H, Karpusas M et al.. (2011) Discovery of a novel class of non-ATP site DFG-out state p38 inhibitors utilizing computationally assisted virtual fragment-based drug design (vFBDD). Bioorg Med Chem Lett, 21 (23): 7155-65. [PMID:22014550]
33. Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev, 22 (2): 153-83. [PMID:11294822]
34. Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. (1995) Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem, 270 (13): 7420-6. [PMID:7535770]
35. Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB. (2007) p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell, 11 (2): 175-89. [PMID:17292828]
36. Roy SM, Grum-Tokars VL, Schavocky JP, Saeed F, Staniszewski A, Teich AF, Arancio O, Bachstetter AD, Webster SJ, Van Eldik LJ et al.. (2015) Targeting human central nervous system protein kinases: An isoform selective p38αMAPK inhibitor that attenuates disease progression in Alzheimer's disease mouse models. ACS Chem Neurosci, 6 (4): 666-80. [PMID:25676389]
37. Tan X, Tester RW, Luedtke GR, Chakravarty S, Mavunkel BJ, Perumattam JJ, Lu Q, Nashashibi I, Jung J, Hu J et al.. (2010) Design and synthesis of piperazine-indole p38 alpha MAP kinase inhibitors with improved pharmacokinetic profiles. Bioorg Med Chem Lett, 20 (3): 828-31. [PMID:20071169]
38. Wang XZ, Ron D. (1996) Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science, 272 (5266): 1347-9. [PMID:8650547]
39. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
40. Xing L, Shieh HS, Selness SR, Devraj RV, Walker JK, Devadas B, Hope HR, Compton RP, Schindler JF, Hirsch JL et al.. (2009) Structural bioinformatics-based prediction of exceptional selectivity of p38 MAP kinase inhibitor PH-797804. Biochemistry, 48 (27): 6402-11. [PMID:19496616]
p38 subfamily: mitogen-activated protein kinase 14. Last modified on 13/10/2023. Accessed on 25/12/2025. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1499.