
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 479 | 11p11.2 | CHRM4 | cholinergic receptor muscarinic 4 | 4,37 |
| Mouse | 7 | 479 | 2 50.63 cM | Chrm4 | cholinergic receptor, muscarinic 4 | 62 |
| Rat | 7 | 478 | 3q24 | Chrm4 | cholinergic receptor, muscarinic 4 | 48,100,115 |
Previous and Unofficial Names  |
|
| HM3 [80-81] | Chrm-4 | cholinergic receptor, muscarinic 4 | cholinergic receptor | |
Database Links  |
|
| Specialist databases | |
| GPCRdb | acm4_human (Hs), acm4_mouse (Mm) |
| Other databases | |
| Alphafold | P08173 (Hs), P32211 (Mm), P08485 (Rn) |
| ChEMBL Target | CHEMBL1821 (Hs), CHEMBL4572 (Mm), CHEMBL317 (Rn) |
| DrugBank Target | P08173 (Hs) |
| Ensembl Gene | ENSG00000180720 (Hs), ENSMUSG00000040495 (Mm), ENSRNOG00000017556 (Rn) |
| Entrez Gene | 1132 (Hs), 12672 (Mm), 25111 (Rn) |
| Human Protein Atlas | ENSG00000180720 (Hs) |
| KEGG Gene | hsa:1132 (Hs), mmu:12672 (Mm), rno:25111 (Rn) |
| OMIM | 118495 (Hs) |
| Pharos | P08173 (Hs) |
| RefSeq Nucleotide | NM_000741 (Hs), NM_007699 (Mm), NM_031547 (Rn) |
| RefSeq Protein | NP_000732 (Hs), NP_031725 (Mm), NP_113735 (Rn) |
| UniProtKB | P08173 (Hs), P32211 (Mm), P08485 (Rn) |
| Wikipedia | CHRM4 (Hs) |
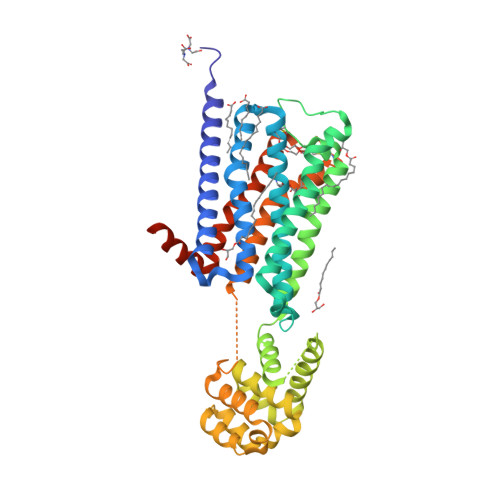
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific agonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The binding data for McN-A-343 [51] is found on rat striatum. Please consult references [6,55,84,108] for further details of the activity of some of the ligands in this list. McN-A-343 and pilocarpine have been found to be partial agonists [55] and full agonists [84,108] at the M4 receptor. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biperiden is an approved drug antagonist of muscarinic acetylcholine receptors. We have tagged the M1 subtype as the drug's primary target as affinity is 10-fold higher at this receptor subtype [3]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulators | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific allosteric modulator tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
| Gi/Go family | Adenylyl cyclase inhibition |
| References: 65,80 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Comments |
| For reviews on muscarinic receptor knockout mice see [10,63,111-113]. |
1. Belov V, Guehl NJ, Duvvuri S, Iredale P, Moon SH, Dhaynaut M, Chakilam S, MacDonagh AC, Rice PA, Yokell DL et al.. (2024) PET imaging of M4 muscarinic acetylcholine receptors in rhesus macaques using [11C]MK-6884: Quantification with kinetic modeling and receptor occupancy by CVL-231 (emraclidine), a novel positive allosteric modulator. J Cereb Blood Flow Metab, 44 (8): 1329-1342. [PMID:38477292]
2. Birdsall NJ, Farries T, Gharagozloo P, Kobayashi S, Lazareno S, Sugimoto M. (1999) Subtype-selective positive cooperative interactions between brucine analogs and acetylcholine at muscarinic receptors: functional studies. Mol Pharmacol, 55 (4): 778-86. [PMID:10101037]
3. Bolden C, Cusack B, Richelson E. (1992) Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther, 260 (2): 576-80. [PMID:1346637]
4. Bonner TI, Modi WS, Seuanez HN, O'Brien SJ. (1991) Chromosomal mapping of the five human genes encoding muscarinic acetylcholine receptors. Cytogenet Cell Genet, 58: 1850-1851.
5. Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, Breininger ML, Gentry PR, Yin H, Jadhav SB et al.. (2008) Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J Pharmacol Exp Ther, 327 (3): 941-53. [PMID:18772318]
6. Bräuner-Osborne H, Ebert B, Brann MR, Falch E, Krogsgaard-Larsen P. (1996) Functional partial agonism at cloned human muscarinic acetylcholine receptors. Eur J Pharmacol, 313 (1-2): 145-50. [PMID:8905341]
7. Bubser M, Bridges TM, Dencker D, Gould RW, Grannan M, Noetzel MJ, Lamsal A, Niswender CM, Daniels JS, Poslusney MS et al.. (2014) Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem Neurosci, 5 (10): 920-42. [PMID:25137629]
8. Buckley NJ, Bonner TI, Buckley CM, Brann MR. (1989) Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol, 35 (4): 469-76. [PMID:2704370]
9. Butler CR, Popiolek M, McAllister LA, LaChapelle EA, Kramer M, Beck EM, Mente S, Brodney MA, Brown M, Gilbert A et al.. (2024) Design and Synthesis of Clinical Candidate PF-06852231 (CVL-231): A Brain Penetrant, Selective, Positive Allosteric Modulator of the M4 Muscarinic Acetylcholine Receptor. J Med Chem, 67 (13): 10831-10847. [PMID:38888621]
10. Bymaster FP, McKinzie DL, Felder CC, Wess J. (2003) Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem Res, 28 (3-4): 437-42. [PMID:12675128]
11. Böhme TM, Keim C, Kreutzmann K, Linder M, Dingermann T, Dannhardt G, Mutschler E, Lambrecht G. (2003) Structure-activity relationships of dimethindene derivatives as new M2-selective muscarinic receptor antagonists. J Med Chem, 46 (5): 856-67. [PMID:12593665]
12. Carr BJ, Mihara K, Ramachandran R, Saifeddine M, Nathanson NM, Stell WK, Hollenberg MD. (2018) Myopia-Inhibiting Concentrations of Muscarinic Receptor Antagonists Block Activation of Alpha2A-Adrenoceptors In Vitro. Invest Ophthalmol Vis Sci, 59 (7): 2778-2791. [PMID:29860464]
13. Cembala TM, Sherwin JD, Tidmarsh MD, Appadu BL, Lambert DG. (1998) Interaction of neuromuscular blocking drugs with recombinant human m1-m5 muscarinic receptors expressed in Chinese hamster ovary cells. Br J Pharmacol, 125 (5): 1088-94. [PMID:9846649]
14. Ch'ng SS, Walker AJ, McCarthy M, Le TK, Thomas N, Gibbons A, Udawela M, Kusljic S, Dean B, Gogos A. (2020) The Impact of Removal of Ovarian Hormones on Cholinergic Muscarinic Receptors: Examining Prepulse Inhibition and Receptor Binding. Brain Sci, 10 (2). [PMID:32079174]
15. Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC, Christopoulos A, Lazareno S, Birdsall NJ, Bymaster FP et al.. (2008) Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci USA, 105 (31): 10978-83. [PMID:18678919]
16. Cheng K, Khurana S, Chen Y, Kennedy RH, Zimniak P, Raufman JP. (2002) Lithocholylcholine, a bile acid/acetylcholine hybrid, is a muscarinic receptor antagonist. J Pharmacol Exp Ther, 303 (1): 29-35. [PMID:12235229]
17. Chernyavsky AI, Arredondo J, Wess J, Karlsson E, Grando SA. (2004) Novel signaling pathways mediating reciprocal control of keratinocyte migration and wound epithelialization through M3 and M4 muscarinic receptors. J Cell Biol, 166 (2): 261-72. [PMID:15263021]
18. Chernyavsky AI, Nguyen VT, Arredondo J, Ndoye A, Zia S, Wess J, Grando SA. (2003) The M4 muscarinic receptor-selective effects on keratinocyte crawling locomotion. Life Sci, 72 (18-19): 2069-73. [PMID:12628458]
19. Christopoulos A, Grant MK, Ayoubzadeh N, Kim ON, Sauerberg P, Jeppesen L, El-Fakahany EE. (2001) Synthesis and pharmacological evaluation of dimeric muscarinic acetylcholine receptor agonists. J Pharmacol Exp Ther, 298 (3): 1260-8. [PMID:11504829]
20. Conn PJ, Jones CK, Lindsley CW. (2009) Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci, 30 (3): 148-55. [PMID:19201489]
21. Croy CH, Chan WY, Castetter AM, Watt ML, Quets AT, Felder CC. (2016) Characterization of PCS1055, a novel muscarinic M4 receptor antagonist. Eur J Pharmacol, 782: 70-6. [PMID:27085897]
22. Croy CH, Schober DA, Xiao H, Quets A, Christopoulos A, Felder CC. (2014) Characterization of the novel positive allosteric modulator, LY2119620, at the muscarinic M(2) and M(4) receptors. Mol Pharmacol, 86 (1): 106-15. [PMID:24807965]
23. D'Agostino G, Barbieri A, Chiossa E, Tonini M. (1997) M4 muscarinic autoreceptor-mediated inhibition of -3H-acetylcholine release in the rat isolated urinary bladder. J Pharmacol Exp Ther, 283 (2): 750-6. [PMID:9353395]
24. D'Agostino G, Bolognesi ML, Lucchelli A, Vicini D, Balestra B, Spelta V, Melchiorre C, Tonini M. (2000) Prejunctional muscarinic inhibitory control of acetylcholine release in the human isolated detrusor: involvement of the M4 receptor subtype. Br J Pharmacol, 129 (3): 493-500. [PMID:10711347]
25. Degroot A, Nomikos GG. (2006) Genetic deletion of muscarinic M4 receptors is anxiolytic in the shock-probe burying model. Eur J Pharmacol, 531 (1-3): 183-6. [PMID:16455072]
26. Del Bello F, Barocelli E, Bertoni S, Bonifazi A, Camalli M, Campi G, Giannella M, Matucci R, Nesi M, Pigini M et al.. (2012) 1,4-dioxane, a suitable scaffold for the development of novel M₃ muscarinic receptor antagonists. J Med Chem, 55 (4): 1783-7. [PMID:22243489]
27. Dörje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann MR. (1991) Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther, 256 (2): 727-33. [PMID:1994002]
28. Ehlert FJ, Delen FM, Yun SH, Friedman DJ, Self DW. (1989) Coupling of subtypes of the muscarinic receptor to adenylate cyclase in the corpus striatum and heart. J Pharmacol Exp Ther, 251 (2): 660-71. [PMID:2810116]
29. Ehlert FJ, Griffin MT, Glidden PF. (1996) The interaction of the enantiomers of aceclidine with subtypes of the muscarinic receptor. J Pharmacol Exp Ther, 279 (3): 1335-44. [PMID:8968358]
30. Esqueda EE, Gerstin Jr EH, Griffin MT, Ehlert FJ. (1996) Stimulation of cyclic AMP accumulation and phosphoinositide hydrolysis by M3 muscarinic receptors in the rat peripheral lung. Biochem Pharmacol, 52 (4): 643-58. [PMID:8759038]
31. Fernandez-Fernandez JM, Wanaverbecq N, Halley P, Caulfield MP, Brown DA. (1999) Selective activation of heterologously expressed G protein-gated K+ channels by M2 muscarinic receptors in rat sympathetic neurones. J Physiol (Lond.), 515 ( Pt 3): 631-7. [PMID:10066893]
32. Fernández-Fernández JM, Abogadie FC, Milligan G, Delmas P, Brown DA. (2001) Multiple pertussis toxin-sensitive G-proteins can couple receptors to GIRK channels in rat sympathetic neurons when expressed heterologously, but only native G(i)-proteins do so in situ. Eur J Neurosci, 14 (2): 283-92. [PMID:11553279]
33. Gentry PR, Kokubo M, Bridges TM, Cho HP, Smith E, Chase P, Hodder PS, Utley TJ, Rajapakse A, Byers F et al.. (2014) Discovery, synthesis and characterization of a highly muscarinic acetylcholine receptor (mAChR)-selective M5-orthosteric antagonist, VU0488130 (ML381): a novel molecular probe. ChemMedChem, 9 (8): 1677-82. [PMID:24692176]
34. Gillberg PG, Sundquist S, Nilvebrant L. (1998) Comparison of the in vitro and in vivo profiles of tolterodine with those of subtype-selective muscarinic receptor antagonists. Eur J Pharmacol, 349 (2-3): 285-92. [PMID:9671109]
35. Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. (1999) Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA, 96 (18): 10483-8. [PMID:10468635]
36. Gomeza J, Zhang L, Kostenis E, Felder CC, Bymaster FP, Brodkin J, Shannon H, Xia B, Duttaroy A, Deng CX et al.. (2001) Generation and pharmacological analysis of M2 and M4 muscarinic receptor knockout mice. Life Sci, 68 (22-23): 2457-66. [PMID:11392613]
37. Grewal RP, Martinez M, Hoehe M, Bonner TI, Gershon ES, Detera-Wadleigh S. (1992) Genetic linkage mapping of the m4 human muscarinic receptor (CHRM4). Genomics, 13 (1): 239-40. [PMID:1577490]
38. Harvey AL, Kornisiuk E, Bradley KN, Cerveñansky C, Durán R, Adrover M, Sánchez G, Jerusalinsky D. (2002) Effects of muscarinic toxins MT1 and MT2 from green mamba on different muscarinic cholinoceptors. Neurochem Res, 27 (11): 1543-54. [PMID:12512959]
39. Hassall CJ, Stanford SC, Burnstock G, Buckley NJ. (1993) Co-expression of four muscarinic receptor genes by the intrinsic neurons of the rat and guinea-pig heart. Neuroscience, 56 (4): 1041-8. [PMID:8284034]
40. Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, Loury D, Eglen RM. (1997) Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol, 120 (8): 1409-18. [PMID:9113359]
41. Hegde SS, Pulido-Rios MT, Luttmann MA, Foley JJ, Hunsberger GE, Steinfeld T, Lee T, Ji Y, Mammen MM, Jasper JR. (2018) Pharmacological properties of revefenacin (TD-4208), a novel, nebulized long-acting, and lung selective muscarinic antagonist, at human recombinant muscarinic receptors and in rat, guinea pig, and human isolated airway tissues. Pharmacol Res Perspect, 6 (3): e00400. [PMID:29736245]
42. Hirose H, Aoki I, Kimura T, Fujikawa T, Numazawa T, Sasaki K, Sato A, Hasegawa T, Nishikibe M, Mitsuya M et al.. (2001) Pharmacological properties of (2R)-N-[1-(6-aminopyridin-2-ylmethyl)piperidin-4-yl]-2-[(1R)-3,3-difluorocyclopentyl]-2-hydroxy-2-phenylacetamide: a novel mucarinic antagonist with M(2)-sparing antagonistic activity. J Pharmacol Exp Ther, 297 (2): 790-7. [PMID:11303071]
43. Hohmann CF, Potter ED, Levey AI. (1995) Development of muscarinic receptor subtypes in the forebrain of the mouse. J Comp Neurol, 358 (1): 88-101. [PMID:7560279]
44. Huang F, Buchwald P, Browne CE, Farag HH, Wu WM, Ji F, Hochhaus G, Bodor N. (2001) Receptor binding studies of soft anticholinergic agents. AAPS PharmSci, 3 (4): E30. [PMID:12049493]
45. Jakubík J, Bacáková L, el-Fakahany EE, Tucek S. (1995) Subtype selectivity of the positive allosteric action of alcuronium at cloned M1-M5 muscarinic acetylcholine receptors. J Pharmacol Exp Ther, 274 (3): 1077-83. [PMID:7562472]
46. Jakubík J, Bacáková L, El-Fakahany EE, Tucek S. (1997) Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Mol Pharmacol, 52 (1): 172-9. [PMID:9224827]
47. Jolkkonen M, van Giersbergen PL, Hellman U, Wernstedt C, Karlsson E. (1994) A toxin from the green mamba Dendroaspis angusticeps: amino acid sequence and selectivity for muscarinic m4 receptors. FEBS Lett, 352 (1): 91-4. [PMID:7925952]
48. Kashihara K, Varga EV, Waite SL, Roeske WR, Yamamura HI. (1992) Cloning of the rat M3, M4 and M5 muscarinic acetylcholine receptor genes by the polymerase chain reaction (PCR) and the pharmacological characterization of the expressed genes. Life Sci, 51 (12): 955-71. [PMID:1325587]
49. Keov P, Valant C, Devine SM, Lane JR, Scammells PJ, Sexton PM, Christopoulos A. (2013) Reverse engineering of the selective agonist TBPB unveils both orthosteric and allosteric modes of action at the M₁ muscarinic acetylcholine receptor. Mol Pharmacol, 84 (3): 425-37. [PMID:23798605]
50. Khattar SK, Bora RS, Priyadarsiny P, Gupta D, Khanna A, Narayanan KL, Babu V, Chugh A, Saini KS. (2006) High level stable expression of pharmacologically active human M1-M5 muscarinic receptor subtypes in mammalian cells. Biotechnol Lett, 28 (2): 121-9. [PMID:16369696]
51. Lambrecht G, Moser U, Grimm U, Pfaff O, Hermanni U, Hildebrandt C, Waelbroeck M, Christophe J, Mutschler E. (1993) New functionally selective muscarinic agonists. Life Sci, 52 (5-6): 481-8. [PMID:7680092]
52. Lazareno S, Birdsall NJ. (1993) Pharmacological characterization of acetylcholine-stimulated [35S]-GTP gamma S binding mediated by human muscarinic m1-m4 receptors: antagonist studies. Br J Pharmacol, 109 (4): 1120-7. [PMID:8401923]
53. Lazareno S, Birdsall NJ. (1995) Detection, quantitation, and verification of allosteric interactions of agents with labeled and unlabeled ligands at G protein-coupled receptors: interactions of strychnine and acetylcholine at muscarinic receptors. Mol Pharmacol, 48 (2): 362-78. [PMID:7651370]
54. Lazareno S, Dolezal V, Popham A, Birdsall NJ. (2004) Thiochrome enhances acetylcholine affinity at muscarinic M4 receptors: receptor subtype selectivity via cooperativity rather than affinity. Mol Pharmacol, 65 (1): 257-66. [PMID:14722259]
55. Lazareno S, Farries T, Birdsall NJ. (1993) Pharmacological characterization of guanine nucleotide exchange reactions in membranes from CHO cells stably transfected with human muscarinic receptors m1-m4. Life Sci, 52 (5-6): 449-56. [PMID:8441327]
56. Lazareno S, Gharagozloo P, Kuonen D, Popham A, Birdsall NJ. (1998) Subtype-selective positive cooperative interactions between brucine analogues and acetylcholine at muscarinic receptors: radioligand binding studies. Mol Pharmacol, 53 (3): 573-89. [PMID:9495826]
57. Lazareno S, Popham A, Birdsall NJ. (2000) Allosteric interactions of staurosporine and other indolocarbazoles with N-[methyl-(3)H]scopolamine and acetylcholine at muscarinic receptor subtypes: identification of a second allosteric site. Mol Pharmacol, 58 (1): 194-207. [PMID:10860942]
58. Lazareno S, Popham A, Birdsall NJ. (2002) Analogs of WIN 62,577 define a second allosteric site on muscarinic receptors. Mol Pharmacol, 62 (6): 1492-505. [PMID:12435818]
59. Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. (1995) Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci, 15 (5 Pt 2): 4077-92. [PMID:7751967]
60. Li W, Wang Y, Lohith TG, Zeng Z, Tong L, Mazzola R, Riffel K, Miller P, Purcell M, Holahan M et al.. (2022) The PET tracer [11C]MK-6884 quantifies M4 muscarinic receptor in rhesus monkeys and patients with Alzheimer's disease. Sci Transl Med, 14 (627): eabg3684. [PMID:35020407]
61. Loudon JM, Bromidge SM, Brown F, Clark MS, Hatcher JP, Hawkins J, Riley GJ, Noy G, Orlek BS. (1997) SB 202026: a novel muscarinic partial agonist with functional selectivity for M1 receptors. J Pharmacol Exp Ther, 283 (3): 1059-68. [PMID:9399977]
62. Matsui M, Araki Y, Karasawa H, Matsubara N, Taketo MM, Seldin MF. (1999) Mapping of five subtype genes for muscarinic acetylcholine receptor to mouse chromosomes. Genes Genet Syst, 74 (1): 15-21. [PMID:10549128]
63. Matsui M, Yamada S, Oki T, Manabe T, Taketo MM, Ehlert FJ. (2004) Functional analysis of muscarinic acetylcholine receptors using knockout mice. Life Sci, 75 (25): 2971-81. [PMID:15474550]
64. McDonald JK, van der Westhuizen ET, Pham V, Thompson G, Felder CC, Paul SM, Thal DM, Christopoulos A, Valant C. (2022) Biased Profile of Xanomeline at the Recombinant Human M4 Muscarinic Acetylcholine Receptor. ACS Chem Neurosci, 13 (8): 1206-1218. [PMID:35380782]
65. Migeon JC, Thomas SL, Nathanson NM. (1995) Differential coupling of m2 and m4 muscarinic receptors to inhibition of adenylyl cyclase by Gi alpha and G(o)alpha subunits. J Biol Chem, 270 (27): 16070-4. [PMID:7608168]
66. Miller JH, Aagaard PJ, Gibson VA, McKinney M. (1992) Binding and functional selectivity of himbacine for cloned and neuronal muscarinic receptors. J Pharmacol Exp Ther, 263 (2): 663-7. [PMID:1331410]
67. Miller JH, Gibson VA, McKinney M. (1991) Binding of [3H]AF-DX 384 to cloned and native muscarinic receptors. J Pharmacol Exp Ther, 259 (2): 601-7. [PMID:1941609]
68. Minarini A, Marucci G, Bellucci C, Giorgi G, Tumiatti V, Bolognesi ML, Matera R, Rosini M, Melchiorre C. (2008) Design, synthesis, and biological evaluation of pirenzepine analogs bearing a 1,2-cyclohexanediamine and perhydroquinoxaline units in exchange for the piperazine ring as antimuscarinics. Bioorg Med Chem, 16 (15): 7311-20. [PMID:18595721]
69. Nag S, Arakawa R, Jia Z, Lachapelle E, Zhang L, Maresca K, Chen L, Jahan M, Mccarthy T, Halldin C. (2023) Characterization of a Novel M4 PAM PET Radioligand [11C]PF06885190 in Nonhuman Primates (NHP). Molecules, 28 (12). [PMID:37375167]
70. Näsman J, Jolkkonen M, Ammoun S, Karlsson E, Akerman KE. (2000) Recombinant expression of a selective blocker of M(1) muscarinic receptors. Biochem Biophys Res Commun, 271 (2): 435-9. [PMID:10799315]
71. Oki T, Takagi Y, Inagaki S, Taketo MM, Manabe T, Matsui M, Yamada S. (2005) Quantitative analysis of binding parameters of [3H]N-methylscopolamine in central nervous system of muscarinic acetylcholine receptor knockout mice. Brain Res Mol Brain Res, 133 (1): 6-11. [PMID:15661360]
72. Olianas MC, Adem A, Karlsson E, Onali P. (1996) Rat striatal muscarinic receptors coupled to the inhibition of adenylyl cyclase activity: potent block by the selective m4 ligand muscarinic toxin 3 (MT3). Br J Pharmacol, 118 (2): 283-8. [PMID:8735628]
73. Olianas MC, Ingianni A, Maullu C, Adem A, Karlsson E, Onali P. (1999) Selectivity profile of muscarinic toxin 3 in functional assays of cloned and native receptors. J Pharmacol Exp Ther, 288 (1): 164-70. [PMID:9862767]
74. Olianas MC, Onali P. (1999) PD 102807, a novel muscarinic M4 receptor antagonist, discriminates between striatal and cortical muscarinic receptors coupled to cyclic AMP. Life Sci, 65 (21): 2233-40. [PMID:10576595]
75. Onali P, Olianas MC. (2002) Muscarinic M4 receptor inhibition of dopamine D1-like receptor signalling in rat nucleus accumbens. Eur J Pharmacol, 448 (2-3): 105-11. [PMID:12144929]
76. Orman B, Sterin-Borda L, Reina S, Borda ES. (2005) Neuronal nitric oxide synthase activity in rat urinary bladder detrusor: participation in M3 and M4 muscarinic receptor function. Auton Autacoid Pharmacol, 25 (3): 93-100. [PMID:15955028]
77. Ozenil M, Pacher K, Balber T, Vraka C, Roller A, Holzer W, Spreitzer H, Mitterhauser M, Wadsak W, Hacker M et al.. (2020) Enhanced arecoline derivatives as muscarinic acetylcholine receptor M1 ligands for potential application as PET radiotracers. Eur J Med Chem, 204: 112623. [PMID:32717485]
78. Pancani T, Foster DJ, Moehle MS, Bichell TJ, Bradley E, Bridges TM, Klar R, Poslusney M, Rook JM, Daniels JS et al.. (2015) Allosteric activation of M4 muscarinic receptors improve behavioral and physiological alterations in early symptomatic YAC128 mice. Proc Natl Acad Sci U S A, 112 (45): 14078-83. [PMID:26508634]
79. Pei XF, Gupta TH, Badio B, Padgett WL, Daly JW. (1998) 6beta-Acetoxynortropane: a potent muscarinic agonist with apparent selectivity toward M2-receptors. J Med Chem, 41 (12): 2047-55. [PMID:9622546]
80. Peralta EG, Ashkenazi A, Winslow JW, Ramachandran J, Capon DJ. (1988) Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature, 334 (6181): 434-7. [PMID:2841607]
81. Peralta EG, Ashkenazi A, Winslow JW, Smith DH, Ramachandran J, Capon DJ. (1987) Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J, 6 (13): 3923-9. [PMID:3443095]
82. Powers AS, Pham V, Burger WAC, Thompson G, Laloudakis Y, Barnes NW, Sexton PM, Paul SM, Christopoulos A, Thal DM et al.. (2023) Structural basis of efficacy-driven ligand selectivity at GPCRs. Nat Chem Biol, 19 (7): 805-814. [PMID:36782010]
83. Preiksaitis HG, Krysiak PS, Chrones T, Rajgopal V, Laurier LG. (2000) Pharmacological and molecular characterization of muscarinic receptor subtypes in human esophageal smooth muscle. J Pharmacol Exp Ther, 295 (3): 879-88. [PMID:11082420]
84. Richards MH, van Giersbergen PL. (1995) Human muscarinic receptors expressed in A9L and CHO cells: activation by full and partial agonists. Br J Pharmacol, 114 (6): 1241-9. [PMID:7620715]
85. Rosenblum K, Futter M, Jones M, Hulme EC, Bliss TV. (2000) ERKI/II regulation by the muscarinic acetylcholine receptors in neurons. J Neurosci, 20 (3): 977-85. [PMID:10648702]
86. Salmon M, Luttmann MA, Foley JJ, Buckley PT, Schmidt DB, Burman M, Webb EF, DeHaas CJ, Kotzer CJ, Barrett VJ et al.. (2013) Pharmacological characterization of GSK573719 (umeclidinium): a novel, long-acting, inhaled antagonist of the muscarinic cholinergic receptors for treatment of pulmonary diseases. J Pharmacol Exp Ther, 345 (2): 260-70. [PMID:23435542]
87. Schrage R, Holze J, Klöckner J, Balkow A, Klause AS, Schmitz AL, De Amici M, Kostenis E, Tränkle C, Holzgrabe U et al.. (2014) New insight into active muscarinic receptors with the novel radioagonist [³H]iperoxo. Biochem Pharmacol, 90 (3): 307-19. [PMID:24863257]
88. Sheffler DJ, Williams R, Bridges TM, Xiang Z, Kane AS, Byun NE, Jadhav S, Mock MM, Zheng F, Lewis LM et al.. (2009) A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Mol Pharmacol, 76 (2): 356-68. [PMID:19407080]
89. Sinha S, Gupta S, Malhotra S, Krishna NS, Meru AV, Babu V, Bansal V, Garg M, Kumar N, Chugh A et al.. (2010) AE9C90CB: a novel, bladder-selective muscarinic receptor antagonist for the treatment of overactive bladder. Br J Pharmacol, 160 (5): 1119-27. [PMID:20590605]
90. Stanton T, Bolden-Watson C, Cusack B, Richelson E. (1993) Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics. Biochem Pharmacol, 45 (11): 2352-4. [PMID:8100134]
91. Stengel PW, Cohen ML. (2002) Muscarinic receptor knockout mice: role of muscarinic acetylcholine receptors M(2), M(3), and M(4) in carbamylcholine-induced gallbladder contractility. J Pharmacol Exp Ther, 301 (2): 643-50. [PMID:11961069]
92. Stocks MJ, Alcaraz L, Bailey A, Bowers K, Donald D, Edwards H, Hunt F, Kindon N, Pairaudeau G, Theaker J et al.. (2010) The discovery of new spirocyclic muscarinic M3 antagonists. Bioorg Med Chem Lett, 20 (24): 7458-61. [PMID:21036043]
93. Sur C, Mallorga PJ, Wittmann M, Jacobson MA, Pascarella D, Williams JB, Brandish PE, Pettibone DJ, Scolnick EM, Conn PJ. (2003) N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-D-aspartate receptor activity. Proc Natl Acad Sci USA, 100 (23): 13674-9. [PMID:14595031]
94. Sykes DA, Dowling MR, Leighton-Davies J, Kent TC, Fawcett L, Renard E, Trifilieff A, Charlton SJ. (2012) The Influence of receptor kinetics on the onset and duration of action and the therapeutic index of NVA237 and tiotropium. J Pharmacol Exp Ther, 343 (2): 520-8. [PMID:22854200]
95. Takeuchi T, Fujinami K, Goto H, Fujita A, Taketo MM, Manabe T, Matsui M, Hata F. (2005) Roles of M2 and M4 muscarinic receptors in regulating acetylcholine release from myenteric neurons of mouse ileum. J Neurophysiol, 93 (5): 2841-8. [PMID:15574798]
96. Tanis SP, Plewe MB, Johnson TW, Butler SL, Dalvie D, DeLisle D, Dress KR, Hu Q, Huang B, Kuehler JE et al.. (2010) Azaindole N-methyl hydroxamic acids as HIV-1 integrase inhibitors-II. The impact of physicochemical properties on ADME and PK. Bioorg Med Chem Lett, 20 (24): 7429-34. [PMID:21036042]
97. Thal DM, Sun B, Feng D, Nawaratne V, Leach K, Felder CC, Bures MG, Evans DA, Weis WI, Bachhawat P et al.. (2016) Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature, 531 (7594): 335-40. [PMID:26958838]
98. Tong L, Li W, Lo MM, Gao X, Wai JM, Rudd M, Tellers D, Joshi A, Zeng Z, Miller P et al.. (2020) Discovery of [11C]MK-6884: A Positron Emission Tomography (PET) Imaging Agent for the Study of M4Muscarinic Receptor Positive Allosteric Modulators (PAMs) in Neurodegenerative Diseases. J Med Chem, 63 (5): 2411-2425. [PMID:32101422]
99. Trendelenburg AU, Gomeza J, Klebroff W, Zhou H, Wess J. (2003) Heterogeneity of presynaptic muscarinic receptors mediating inhibition of sympathetic transmitter release: a study with M2- and M4-receptor-deficient mice. Br J Pharmacol, 138 (3): 469-80. [PMID:12569072]
100. Tseng J, Erbe CB, Kwitek AE, Jacob HJ, Popper P, Wackym PA. (2002) Radiation hybrid mapping of five muscarinic acetylcholine receptor subtype genes in Rattus norvegicus. Hear Res, 174 (1-2): 86-92. [PMID:12433399]
101. Tyagi S, Tyagi P, Van-le S, Yoshimura N, Chancellor MB, de Miguel F. (2006) Qualitative and quantitative expression profile of muscarinic receptors in human urothelium and detrusor. J Urol, 176 (4 Pt 1): 1673-8. [PMID:16952712]
102. Tzavara ET, Bymaster FP, Felder CC, Wade M, Gomeza J, Wess J, McKinzie DL, Nomikos GG. (2003) Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol Psychiatry, 8 (7): 673-9. [PMID:12874603]
103. Valuskova P, Farar V, Forczek S, Krizova I, Myslivecek J. (2018) Autoradiography of 3H-pirenzepine and 3H-AFDX-384 in Mouse Brain Regions: Possible Insights into M1, M2, and M4 Muscarinic Receptors Distribution. Front Pharmacol, 9: 124. [PMID:29515448]
104. Vilaró MT, Palacios JM, Mengod G. (1994) Multiplicity of muscarinic autoreceptor subtypes? Comparison of the distribution of cholinergic cells and cells containing mRNA for five subtypes of muscarinic receptors in the rat brain. Brain Res Mol Brain Res, 21 (1-2): 30-46. [PMID:8164520]
105. Vuckovic Z, Wang J, Pham V, Mobbs JI, Belousoff MJ, Bhattarai A, Burger WAC, Thompson G, Yeasmin M, Nawaratne V et al.. (2023) Pharmacological hallmarks of allostery at the M4 muscarinic receptor elucidated through structure and dynamics. Elife, 12. [PMID:37248726]
106. Wackym PA, Chen CT, Ishiyama A, Pettis RM, López IA, Hoffman L. (1996) Muscarinic acetylcholine receptor subtype mRNAs in the human and rat vestibular periphery. Cell Biol Int, 20 (3): 187-92. [PMID:8673067]
107. Waelbroeck M, De Neef P, Domenach V, Vandermeers-Piret MC, Vandermeers A. (1996) Binding of the labelled muscarinic toxin 125I-MT1 to rat brain muscarinic M1 receptors. Eur J Pharmacol, 305 (1-3): 187-92. [PMID:8813552]
108. Wang SZ, el-Fakahany EE. (1993) Application of transfected cell lines in studies of functional receptor subtype selectivity of muscarinic agonists. J Pharmacol Exp Ther, 266 (1): 237-43. [PMID:7687290]
109. Watson J, Brough S, Coldwell MC, Gager T, Ho M, Hunter AJ, Jerman J, Middlemiss DN, Riley GJ, Brown AM. (1998) Functional effects of the muscarinic receptor agonist, xanomeline, at 5-HT1 and 5-HT2 receptors. Br J Pharmacol, 125 (7): 1413-20. [PMID:9884068]
110. Weiner DM, Levey AI, Brann MR. (1990) Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci USA, 87 (18): 7050-4. [PMID:2402490]
111. Wess J. (2003) Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol Sci, 24 (8): 414-20. [PMID:12915051]
112. Wess J. (2004) Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol, 44: 423-50. [PMID:14744253]
113. Wess J, Duttaroy A, Zhang W, Gomeza J, Cui Y, Miyakawa T, Bymaster FP, McKinzie L, Felder CC, Lamping KG et al.. (2003) M1-M5 muscarinic receptor knockout mice as novel tools to study the physiological roles of the muscarinic cholinergic system. Recept Channels, 9 (4): 279-90. [PMID:12893539]
114. Wess J, Lambrecht G, Mutschler E, Brann MR, Dörje F. (1991) Selectivity profile of the novel muscarinic antagonist UH-AH 37 determined by the use of cloned receptors and isolated tissue preparations. Br J Pharmacol, 102 (1): 246-50. [PMID:2043926]
115. Wood IC, Roopra A, Harrington C, Buckley NJ. (1995) Structure of the m4 cholinergic muscarinic receptor gene and its promoter. J Biol Chem, 270 (52): 30933-40. [PMID:8537349]
116. Wood MD, Murkitt KL, Ho M, Watson JM, Brown F, Hunter AJ, Middlemiss DN. (1999) Functional comparison of muscarinic partial agonists at muscarinic receptor subtypes hM1, hM2, hM3, hM4 and hM5 using microphysiometry. Br J Pharmacol, 126 (7): 1620-4. [PMID:10323594]
117. Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, Weisstein JS, Spagnola BV, Wolfe BB. (1993) Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: distribution of m4 and m5 receptors in rat brain. Mol Pharmacol, 43 (2): 149-57. [PMID:8429821]
118. Zhang W, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. (2002) Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J Neurosci, 22 (5): 1709-17. [PMID:11880500]
119. Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. (2002) Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci, 22 (15): 6347-52. [PMID:12151512]