
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
 target has curated data in GtoImmuPdb
target has curated data in GtoImmuPdb
Target id: 2047
Nomenclature: Janus kinase 1
Abbreviated Name: JAK1
Family: Janus kinase (JakA) family
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 1154 | 1p31.3 | JAK1 | Janus kinase 1 | |
| Mouse | - | 1153 | 4 2.05 cM | Jak1 | Janus kinase 1 | |
| Rat | - | 1153 | 5 q31.3-q35 | Jak1 | Janus kinase 1 | |
Previous and Unofficial Names  |
| JAK1A | JAK1B | JTK3 |
Database Links  |
|
| Alphafold | P23458 (Hs), P52332 (Mm) |
| BRENDA | 2.7.10.2 |
| CATH/Gene3D | 3.30.505.10, 1.20.80.10 |
| ChEMBL Target | CHEMBL2835 (Hs), CHEMBL2968 (Mm) |
| DrugBank Target | P23458 (Hs) |
| Ensembl Gene | ENSG00000162434 (Hs), ENSMUSG00000028530 (Mm), ENSRNOG00000011157 (Rn) |
| Entrez Gene | 3716 (Hs), 16451 (Mm), 84598 (Rn) |
| Human Protein Atlas | ENSG00000162434 (Hs) |
| KEGG Enzyme | 2.7.10.2 |
| KEGG Gene | hsa:3716 (Hs), mmu:16451 (Mm), rno:84598 (Rn) |
| OMIM | 147795 (Hs) |
| Pharos | P23458 (Hs) |
| RefSeq Nucleotide | NM_002227 (Hs), NM_146145 (Mm), NM_053466 (Rn) |
| RefSeq Protein | NP_002218 (Hs), NP_666257 (Mm), NP_445918 (Rn) |
| UniProtKB | P23458 (Hs), P52332 (Mm) |
| Wikipedia | JAK1 (Hs) |
Selected 3D Structures  |
|||||||||||||
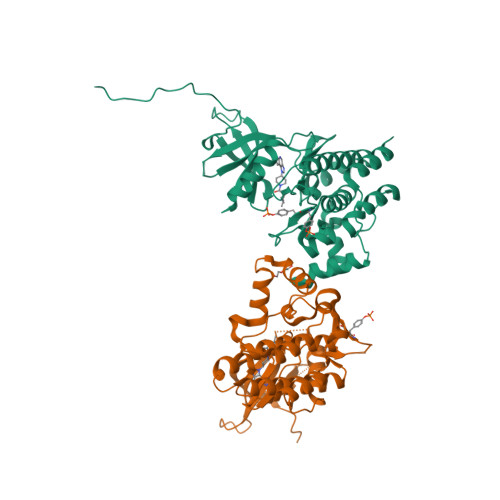
|
|
||||||||||||

|
|
||||||||||||
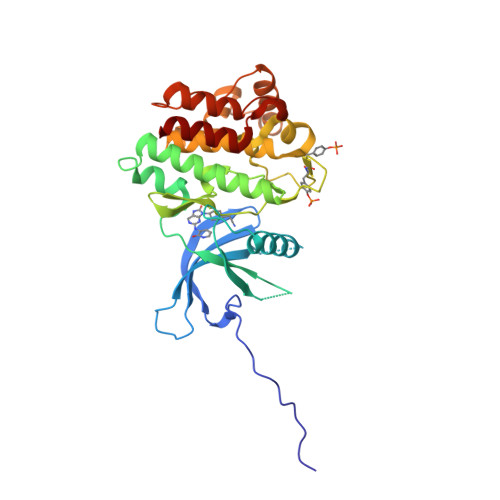
|
|
||||||||||||
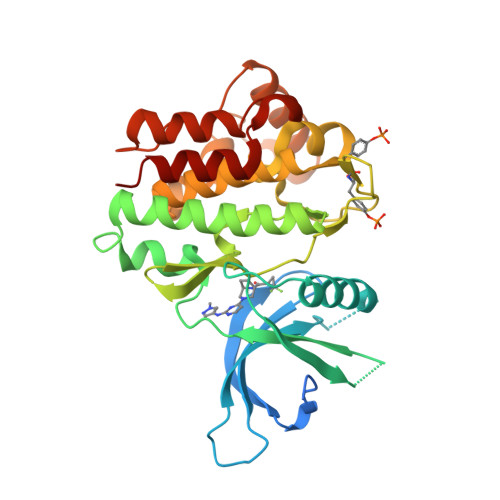
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inhibitor Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Binding to JAK1 pseudokinase domain, although potent, produces low functional activity in a JAK1/JAK3 dependent IL-2 stimulated cellular assay [58]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Allosteric Modulators | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Other Binding Ligands | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 13,56 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: JAK1(JH1domain-catalytic) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: JAK1(JH2domain-pseudokinase) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: ...1 |
 
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: nd/JAK1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| The JAK1 tyrosine kinase is crucial for signaling of certain type I and type II cytokines, via receptors belonging to the IL-2, IL-4 and IL-6 receptor families as well as neurotrophin-1 and leptin receptors (all type I cytokine receptors). JAK1 is also involved in signalling via type II IL-10 family receptors, and receptors for type I and type II interferons (IFN-α/β and IFN-γ respectively). |
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
1. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377]
2. Anderson DR, Hockerman SL, Blinn JR, Jacobsen EJ. (2019) Substituted pyrrolopyrimidine jak inhibitors and methods of making and using the same. Patent number: WO2019090158A1. Assignee: Aclaris Therapeutics, Inc.. Priority date: 02/10/2029. Publication date: 02/08/2029.
3. Asshoff M, Petzer V, Warr MR, Haschka D, Tymoszuk P, Demetz E, Seifert M, Posch W, Nairz M, Maciejewski P et al.. (2017) Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood, 129 (13): 1823-1830. [PMID:28188131]
4. Bach J, Eastwood P, González J, Gómez E, Alonso JA, Fonquerna S, Lozoya E, Orellana A, Maldonado M, Calaf E et al.. (2019) Identification of 2-Imidazopyridine and 2-Aminopyridone Purinones as Potent Pan-Janus Kinase (JAK) Inhibitors for the Inhaled Treatment of Respiratory Diseases. J Med Chem, 62 (20): 9045-9060. [PMID:31609613]
5. Berlinski PJ, Birchmeier MJ, Bowman JW, Gonzales AJ, Kamerling SG, Mann DW, Mitton-Fry MJ. (2010) Pyrrolo[2,3-d]pyrimidine compounds. Patent number: WO2010020905. Assignee: Pfizer Inc.. Priority date: 20/08/2008. Publication date: 25/02/2010.
6. Blanc J. (2010) Novel compound useful for the treatment of degenerative and inflammatory diseases. Patent number: WO2010149771. Assignee: Galapagos Nv, Menet, Christel Jeanne Marie. Priority date: 26/06/2009. Publication date: 29/12/2010.
7. Brown GR, Bamford AM, Bowyer J, James DS, Rankine N, Tang E, Torr V, Culbert EJ. (2000) Naphthyl ketones: a new class of Janus kinase 3 inhibitors. Bioorg Med Chem Lett, 10 (6): 575-9. [PMID:10741557]
8. Casimiro-Garcia A, Trujillo JI, Vajdos F, Juba B, Banker ME, Aulabaugh A, Balbo P, Bauman J, Chrencik J, Coe JW et al.. (2018) Identification of Cyanamide-Based Janus Kinase 3 (JAK3) Covalent Inhibitors. J Med Chem, 61 (23): 10665-10699. [PMID:30423248]
9. Chang Y, Min J, Jarusiewicz JA, Actis M, Yu-Chen Bradford S, Mayasundari A, Yang L, Chepyala D, Alcock LJ, Roberts KG et al.. (2021) Degradation of Janus kinases in CRLF2-rearranged acute lymphoblastic leukemia. Blood, 138 (23): 2313-2326. [PMID:34110416]
10. Changelian PS, Moshinsky D, Kuhn CF, Flanagan ME, Munchhof MJ, Harris TM, Whipple DA, Doty JL, Sun J, Kent CR et al.. (2008) The specificity of JAK3 kinase inhibitors. Blood, 111 (4): 2155-7. [PMID:18094329]
11. Clark JD, Flanagan ME, Telliez JB. (2014) Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem, 57 (12): 5023-38. [PMID:24417533]
12. Coffey G, Betz A, DeGuzman F, Pak Y, Inagaki M, Baker DC, Hollenbach SJ, Pandey A, Sinha U. (2014) The novel kinase inhibitor PRT062070 (Cerdulatinib) demonstrates efficacy in models of autoimmunity and B-cell cancer. J Pharmacol Exp Ther, 351 (3): 538-48. [PMID:25253883]
13. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
14. Dengler HS, Wu X, Peng I, Rinderknecht CH, Kwon Y, Suto E, Kohli PB, Liimatta M, Barrett K, Lloyd J et al.. (2018) Lung-restricted inhibition of Janus kinase 1 is effective in rodent models of asthma. Sci Transl Med, 10 (468): eaao2151. [PMID:30463918]
15. Elsayed MSA, Nielsen JJ, Park S, Park J, Liu Q, Kim CH, Pommier Y, Agama K, Low PS, Cushman M. (2018) Application of Sequential Palladium Catalysis for the Discovery of Janus Kinase Inhibitors in the Benzo[ c]pyrrolo[2,3- h][1,6]naphthyridin-5-one (BPN) Series. J Med Chem, 61 (23): 10440-10462. [PMID:30460842]
16. Fensome A, Ambler CM, Arnold E, Banker ME, Brown MF, Chrencik J, Clark JD, Dowty ME, Efremov IV, Flick A et al.. (2018) Dual Inhibition of TYK2 and JAK1 for the Treatment of Autoimmune Diseases: Discovery of (( S)-2,2-Difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl)amino)pyrimidin-4-yl)-3,8-diazabicyclo[3.2.1]octan-8-yl)methanone (PF-06700841). J Med Chem, 61 (19): 8597-8612. [PMID:30113844]
17. Forsyth T, Kearney PC, Kim BG, Johnson HW, Aay N, Arcalas A, Brown DS, Chan V, Chen J, Du H et al.. (2012) SAR and in vivo evaluation of 4-aryl-2-aminoalkylpyrimidines as potent and selective Janus kinase 2 (JAK2) inhibitors. Bioorg Med Chem Lett, 22 (24): 7653-8. [PMID:23127890]
18. Fridman JS, Scherle PA, Collins R, Burn TC, Li Y, Li J, Covington MB, Thomas B, Collier P, Favata MF et al.. (2010) Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol, 184 (9): 5298-307. [PMID:20363976]
19. Gerstenberger BS, Ambler C, Arnold EP, Banker ME, Brown MF, Clark JD, Dermenci A, Dowty ME, Fensome A, Fish S et al.. (2020) Discovery of Tyrosine Kinase 2 (TYK2) Inhibitor (PF-06826647) for the Treatment of Autoimmune Diseases. J Med Chem, 63 (22): 13561-13577. [PMID:32787094]
20. Goh KC, Novotny-Diermayr V, Hart S, Ong LC, Loh YK, Cheong A, Tan YC, Hu C, Jayaraman R, William AD et al.. (2012) TG02, a novel oral multi-kinase inhibitor of CDKs, JAK2 and FLT3 with potent anti-leukemic properties. Leukemia, 26 (2): 236-43. [PMID:21860433]
21. Hanan EJ, Liang J, Wang X, Blake RA, Blaquiere N, Staben ST. (2020) Monomeric Targeted Protein Degraders. J Med Chem, 63 (20): 11330-11361. [PMID:32352776]
22. Hanan EJ, van Abbema A, Barrett K, Blair WS, Blaney J, Chang C, Eigenbrot C, Flynn S, Gibbons P, Hurley CA et al.. (2012) Discovery of potent and selective pyrazolopyrimidine janus kinase 2 inhibitors. J Med Chem, 55 (22): 10090-107. [PMID:23061660]
23. Huang T, Xue C-B, Wang A, Kong L, Ye HF, Yao W, Rodgers JD, Shepard S, Wang H, Shao L et al.. (2011) Piperidin-4-yl azetidine derivatives as jak1 inhibitors. Patent number: WO2011112662. Assignee: Incyte Corporation. Priority date: 10/03/2010. Publication date: 15/09/2011.
24. Hudson R, Kozak J, Fatheree PR, Podesto DD, Brandt GEL, Fleury M, Beausoleil A-M, Huang X, Thalladi VR. (2016) Naphthyridine compounds as jak kinase inhibitors. Patent number: WO2016191524A1. Assignee: Theravance Biopharma R&D. Priority date: 28/05/2015. Publication date: 01/12/2016.
25. Ioannidis S, Lamb ML, Wang T, Almeida L, Block MH, Davies AM, Peng B, Su M, Zhang HJ, Hoffmann E et al.. (2011) Discovery of 5-chloro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]-N4-(5-methyl-1H-pyrazol-3-yl)pyrimidine-2,4-diamine (AZD1480) as a novel inhibitor of the Jak/Stat pathway. J Med Chem, 54 (1): 262-76. [PMID:21138246]
26. Ito M, Yamazaki S, Yamagami K, Kuno M, Morita Y, Okuma K, Nakamura K, Chida N, Inami M, Inoue T et al.. (2017) A novel JAK inhibitor, peficitinib, demonstrates potent efficacy in a rat adjuvant-induced arthritis model. J Pharmacol Sci, 133 (1): 25-33. [PMID:28117214]
27. Jarusiewicz JA, Jeon JY, Connelly MC, Chen Y, Yang L, Baker SD, Guy RK. (2017) Discovery of a Diaminopyrimidine FLT3 Inhibitor Active against Acute Myeloid Leukemia. ACS Omega, 2 (5): 1985-2009. [PMID:28580438]
28. Jones P, Storer RI, Sabnis YA, Wakenhut FM, Whitlock GA, England KS, Mukaiyama T, Dehnhardt CM, Coe JW, Kortum SW et al.. (2017) Design and Synthesis of a Pan-Janus Kinase Inhibitor Clinical Candidate (PF-06263276) Suitable for Inhaled and Topical Delivery for the Treatment of Inflammatory Diseases of the Lungs and Skin. J Med Chem, 60 (2): 767-786. [PMID:27983835]
29. Koudriakova T, Kreutter K, Leonard K, Rizzolio M, Smith RC, Tichenor MS, Wang A. (2018) Small molecule inhibitors of the JAK family of kinases. Patent number: WO2018112379A1. Assignee: Janssen Pharmaceutica. Priority date: 16/12/2016. Publication date: 21/06/2018.
30. Kulagowski JJ, Blair W, Bull RJ, Chang C, Deshmukh G, Dyke HJ, Eigenbrot C, Ghilardi N, Gibbons P, Harrison TK et al.. (2012) Identification of imidazo-pyrrolopyridines as novel and potent JAK1 inhibitors. J Med Chem, 55 (12): 5901-21. [PMID:22591402]
31. Li Y-L, Zhuo J, Qian D-Q, Mei S, Cao G, Pan Y, Li Q, Jia Z. (2021) Bipyrazole derivatives as jak inhibitors. Patent number: US20210238168A1. Assignee: Incyte Corp. Priority date: 17/05/2013. Publication date: 05/08/2021.
32. Liang C. (2020) JAK1 selective inhibitors and uses thereof. Patent number: US10738060B2. Assignee: Hangzhou Highlightll Pharmaceutical Co Ltd, Tll Pharmaceutical LLC. Priority date: 30/09/2017. Publication date: 11/08/2020.
33. Liang X, Xie Y, Liu X, Xu H, Ren H, Tang S, Liu Q, Huang M, Shao X, Li C et al.. (2022) Discovery of Novel Imidazo[4,5-c]quinoline Derivatives to Treat Inflammatory Bowel Disease (IBD) by Inhibiting Multiple Proinflammatory Signaling Pathways and Restoring Intestinal Homeostasis. J Med Chem, 65 (18): 11949-11969. [PMID:36053746]
34. Long DD, Smith C, Thompson C. (2020) DIMETHYL AMINO AZETIDINE AMIDES AS JAK INHIBITORS. Patent number: WO/2020/051105. Assignee: THERAVANCE BIOPHARMA. Priority date: 04/09/2018. Publication date: 12/03/2020.
35. Lu T. (2022) Jak inhibitor and preparation method therefor. Patent number: US20220106319A1. Assignee: Felicamed Biotechnology Co Ltd. Priority date: 23/12/2019. Publication date: 07/04/2022.
36. Malerich JP, Lam JS, Hart B, Fine RM, Klebansky B, Tanga MJ, D'Andrea A. (2010) Diamino-1,2,4-triazole derivatives are selective inhibitors of TYK2 and JAK1 over JAK2 and JAK3. Bioorg Med Chem Lett, 20 (24): 7454-7. [PMID:21106455]
37. Nakaya Y, Shide K, Niwa T, Homan J, Sugahara S, Horio T, Kuramoto K, Kotera T, Shibayama H, Hori K et al.. (2011) Efficacy of NS-018, a potent and selective JAK2/Src inhibitor, in primary cells and mouse models of myeloproliferative neoplasms. Blood Cancer J, 1 (7): e29. [PMID:22829185]
38. Nilsson KM, Astrand ABM, Berggren AIK, Johansson JR, Lepisto MJ, Kawatkar SP, Su Q, Kettle JG. (2018) Jak1 selective inhibitors. Patent number: WO2018134213A1. Assignee: Astrazeneca Ab. Priority date: 17/01/2017. Publication date: 26/07/2018.
39. Noji S, Hara Y, Miura T, Yamanaka H, Maeda K, Hori A, Yamamoto H, Obika S, Inoue M, Hase Y et al.. (2020) Discovery of a Janus Kinase Inhibitor Bearing a Highly Three-Dimensional Spiro Scaffold: JTE-052 (Delgocitinib) as a New Dermatological Agent to Treat Inflammatory Skin Disorders. J Med Chem, 63 (13): 7163-7185. [PMID:32511913]
40. Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. (2009) CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia, 23 (8): 1441-5. [PMID:19295546]
41. Purandare AV, McDevitt TM, Wan H, You D, Penhallow B, Han X, Vuppugalla R, Zhang Y, Ruepp SU, Trainor GL et al.. (2012) Characterization of BMS-911543, a functionally selective small-molecule inhibitor of JAK2. Leukemia, 26 (2): 280-8. [PMID:22015772]
42. Qiu Q, Chi F, Zhou D, Xie Z, Liu Y, Wu H, Yin Z, Shi W, Qian H. (2023) Exploration of Janus Kinase (JAK) and Histone Deacetylase (HDAC) Bispecific Inhibitors Based on the Moiety of Fedratinib for Treatment of Both Hematologic Malignancies and Solid Cancers. J Med Chem, 66 (8): 5753-5773. [PMID:37057760]
43. Quintás-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA, Caulder E, Wen X, Li Y, Waeltz P et al.. (2010) Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood, 115 (15): 3109-17. [PMID:20130243]
44. Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, King KL, Sheehan KC, Yin L, Pennica D et al.. (1998) Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell, 93 (3): 373-83. [PMID:9590172]
45. Su Q, Banks E, Bebernitz G, Bell K, Borenstein CF, Chen H, Chuaqui CE, Deng N, Ferguson AD, Kawatkar S et al.. (2020) Discovery of (2R)-N-[3-[2-[(3-Methoxy-1-methyl-pyrazol-4-yl)amino]pyrimidin-4-yl]-1H-indol-7-yl]-2-(4-methylpiperazin-1-yl)propenamide (AZD4205) as a Potent and Selective Janus Kinase 1 Inhibitor. J Med Chem, 63 (9): 4517-4527. [PMID:32297743]
46. Su Q, Ioannidis S, Chuaqui C, Almeida L, Alimzhanov M, Bebernitz G, Bell K, Block M, Howard T, Huang S et al.. (2014) Discovery of 1-methyl-1H-imidazole derivatives as potent Jak2 inhibitors. J Med Chem, 57 (1): 144-58. [PMID:24359159]
47. Tanimoto A, Ogawa Y, Oki C, Kimoto Y, Nozawa K, Amano W, Noji S, Shiozaki M, Matsuo A, Shinozaki Y et al.. (2015) Pharmacological properties of JTE-052: a novel potent JAK inhibitor that suppresses various inflammatory responses in vitro and in vivo. Inflamm Res, 64 (1): 41-51. [PMID:25387665]
48. Thoma G, Duthaler RO, Waelchli R, Hauchard A, Bruno S, Strittmatter-Keller U, Orjuela Leon A, Viebrock S, Aichholz R, Beltz K et al.. (2023) Discovery and Characterization of the Topical Soft JAK Inhibitor CEE321 for Atopic Dermatitis. J Med Chem, 66 (3): 2161-2168. [PMID:36657024]
49. Thorarensen A, Dowty ME, Banker ME, Juba B, Jussif J, Lin T, Vincent F, Czerwinski RM, Casimiro-Garcia A, Unwalla R et al.. (2017) Design of a Janus Kinase 3 (JAK3) Specific Inhibitor 1-((2S,5R)-5-((7H-Pyrrolo[2,3-d]pyrimidin-4-yl)amino)-2-methylpiperidin-1-yl)prop-2-en-1-one (PF-06651600) Allowing for the Interrogation of JAK3 Signaling in Humans. J Med Chem, 60 (5): 1971-1993. [PMID:28139931]
50. Van Rompaey L, Galien R, van der Aar EM, Clement-Lacroix P, Nelles L, Smets B, Lepescheux L, Christophe T, Conrath K, Vandeghinste N et al.. (2013) Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J Immunol, 191 (7): 3568-77. [PMID:24006460]
51. Vazquez ML, Kaila N, Strohbach JW, Trzupek JD, Brown MF, Flanagan ME, Mitton-Fry MJ, Johnson TA, TenBrink RE, Arnold EP et al.. (2018) Identification of N-{cis-3-[Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}propane-1-sulfonamide (PF-04965842): A Selective JAK1 Clinical Candidate for the Treatment of Autoimmune Diseases. J Med Chem, 61 (3): 1130-1152. [PMID:29298069]
52. Voss JW, Camp HS, Padley RJ. (2015) Jak1 selective inhibitor and uses thereof. Patent number: WO2015061665. Assignee: Abbvie Inc.. Priority date: 24/10/2013. Publication date: 30/04/2015.
53. Wagner AT, Cassella JV, Graham PB, Braman V, Uttamsingh V, Von Hehn J, Hamilton CE. (2017) Treatment of hair loss disorders with deuterated jak inhibitors. Patent number: WO2017192905A1. Assignee: Concert Pharmaceuticals. Priority date: 04/05/2016. Publication date: 09/11/2017.
54. Wan Z, Vazquez ML. (2022) Tricyclic janus kinase 1 inhibitors, and compositions and methods thereof. Patent number: US20220009927. Assignee: Lynk Pharmaceuticals Co Ltd. Priority date: 01/11/2019. Publication date: 13/01/2022.
55. William AD, Lee AC, Blanchard S, Poulsen A, Teo EL, Nagaraj H, Tan E, Chen D, Williams M, Sun ET et al.. (2011) Discovery of the macrocycle 11-(2-pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a potent Janus kinase 2/fms-like tyrosine kinase-3 (JAK2/FLT3) inhibitor for the treatment of myelofibrosis and lymphoma. J Med Chem, 54 (13): 4638-58. [PMID:21604762]
56. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
57. Works MG, Yin F, Yin CC, Yiu Y, Shew K, Tran TT, Dunlap N, Lam J, Mitchell T, Reader J et al.. (2014) Inhibition of TYK2 and JAK1 ameliorates imiquimod-induced psoriasis-like dermatitis by inhibiting IL-22 and the IL-23/IL-17 axis. J Immunol, 193 (7): 3278-87. [PMID:25156366]
58. Wrobleski ST, Moslin R, Lin S, Zhang Y, Spergel S, Kempson J, Tokarski JS, Strnad J, Zupa-Fernandez A, Cheng L et al.. (2019) Highly Selective Inhibition of Tyrosine Kinase 2 (TYK2) for the Treatment of Autoimmune Diseases: Discovery of the Allosteric Inhibitor BMS-986165. J Med Chem, 62 (20): 8973-8995. [PMID:31318208]
59. Wu H, Huang Q, Qi Z, Chen Y, Wang A, Chen C, Liang Q, Wang J, Chen W, Dong J et al.. (2017) Irreversible inhibition of BTK kinase by a novel highly selective inhibitor CHMFL-BTK-11 suppresses inflammatory response in rheumatoid arthritis model. Sci Rep, 7 (1): 466. [PMID:28352114]
60. Yang T, Hu M, Chen Y, Xiang M, Tang M, Qi W, Shi M, He J, Yuan X, Zhang C et al.. (2020) N-(Pyrimidin-2-yl)-1,2,3,4-tetrahydroisoquinolin-6-amine Derivatives as Selective Janus Kinase 2 Inhibitors for the Treatment of Myeloproliferative Neoplasms. J Med Chem, 63 (23): 14921-14936. [PMID:33256400]
61. Yang T, Hu M, Qi W, Yang Z, Tang M, He J, Chen Y, Bai P, Yuan X, Zhang C et al.. (2019) Discovery of Potent and Orally Effective Dual Janus Kinase 2/FLT3 Inhibitors for the Treatment of Acute Myelogenous Leukemia and Myeloproliferative Neoplasms. J Med Chem, 62 (22): 10305-10320. [PMID:31670517]
62. Zhang X, Dong Q, Liu B, Zhu Y, Li X, Lan J. (2013) Pyrrole six-membered heteroaryl ring derivative, preparation method therefor, and medicinal uses thereof. Patent number: WO2013091539A1. Assignee: Jiangsu Hengrui Medicine Co., Ltd., Shanghai Hengrui Medicine Co., Ltd.. Priority date: 21/12/2011. Publication date: 27/06/2013.
63. Zhao C, Zhang Y, Zhang J, Li S, Liu M, Geng Y, Liu F, Chai Q, Meng H, Li M et al.. (2023) Discovery of Novel Fedratinib-Based HDAC/JAK/BRD4 Triple Inhibitors with Remarkable Antitumor Activity against Triple Negative Breast Cancer. J Med Chem, 66 (20): 14150-14174. [PMID:37796543]
64. Zhou S, Mao W, Su Y, Zheng X, Qian W, Shen M, Shan N, Li Y, Wang D, Wu S et al.. (2022) Identification of TUL01101: A Novel Potent and Selective JAK1 Inhibitor for the Treatment of Rheumatoid Arthritis. J Med Chem, 65 (24): 16716-16740. [PMID:36512734]
65. Zhu Y, Ma Y, Zu W, Song J, Wang H, Zhong Y, Li H, Zhang Y, Gao Q, Kong B et al.. (2020) Identification of N-Phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine Derivatives as Novel, Potent, and Selective NF-κB Inducing Kinase (NIK) Inhibitors for the Treatment of Psoriasis. J Med Chem, 63 (13): 6748-6773. [PMID:32479083]
Janus kinase (JakA) family: Janus kinase 1. Last modified on 28/07/2025. Accessed on 02/02/2026. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2047.