|
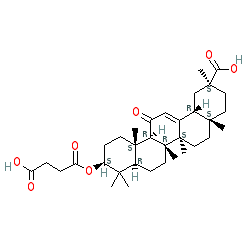
Comment: Carbenoxolone is derived from licorice root, and has a steroid-like chemical structure. Formulations used clinically typically contain carbenoxolone sodium.
|
|
2D Structure
|
|
Physico-chemical Properties
|
|
|
Hydrogen bond acceptors
|
7
|
|
Hydrogen bond donors
|
2
|
|
Rotatable bonds
|
6
|
|
Topological polar surface area
|
117.97
|
|
Molecular weight
|
570.36
|
|
XLogP
|
6.86
|
|
No. Lipinski's rules broken
|
1
|
Generated using the Chemistry Development Kit (CDK) (Willighagen EL et al. Journal of Cheminformatics vol. 9:33. 2017, doi:10.1186/s13321-017-0220-4; https://cdk.github.io/)
|
SMILES / InChI / InChIKey
|
|
|
Canonical SMILES
|
O=C(OC1CCC2(C(C1(C)C)CCC1(C2C(=O)C=C2C1(C)CCC1(C2CC(C)(CC1)C(=O)O)C)C)C)CCC(=O)O
|
|
Isomeric SMILES
|
O=C(O[C@H]1CC[C@]2([C@H](C1(C)C)CC[C@@]1([C@@H]2C(=O)C=C2[C@@]1(C)CC[C@@]1([C@H]2C[C@](C)(CC1)C(=O)O)C)C)C)CCC(=O)O
|
|
InChI
|
InChI=1S/C34H50O7/c1-29(2)23-10-13-34(7)27(32(23,5)12-11-24(29)41-26(38)9-8-25(36)37)22(35)18-20-21-19-31(4,28(39)40)15-14-30(21,3)16-17-33(20,34)6/h18,21,23-24,27H,8-17,19H2,1-7H3,(H,36,37)(H,39,40)/t21-,23-,24-,27+,30+,31-,32-,33+,34+/m0/s1
|
|
InChI Key
|
OBZHEBDUNPOCJG-WBXJDKIVSA-N
|
Generated using the Chemistry Development Kit (CDK) (Willighagen EL et al. Journal of Cheminformatics vol. 9:33. 2017, doi:10.1186/s13321-017-0220-4; https://cdk.github.io/)
|
|


