
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
 target has curated data in GtoImmuPdb
target has curated data in GtoImmuPdb
Target id: 2049
Nomenclature: Janus kinase 3
Abbreviated Name: JAK3
Family: Janus kinase (JakA) family
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 1124 | 19p13.11 | JAK3 | Janus kinase 3 | |
| Mouse | - | 1100 | 8 34.43 cM | Jak3 | Janus kinase 3 | |
| Rat | - | 1100 | 16p14 | Jak3 | Janus kinase 3 | |
Previous and Unofficial Names  |
| JAKL | leukocyte Janus kinase | LJAK |
Database Links  |
|
| Alphafold | P52333 (Hs), Q62137 (Mm), Q63272 (Rn) |
| BRENDA | 2.7.10.2 |
| CATH/Gene3D | 3.30.505.10, 1.20.80.10 |
| ChEMBL Target | CHEMBL2148 (Hs), CHEMBL5250 (Mm), CHEMBL4295857 (Rn) |
| DrugBank Target | P52333 (Hs) |
| Ensembl Gene | ENSG00000105639 (Hs), ENSMUSG00000031805 (Mm), ENSRNOG00000018669 (Rn) |
| Entrez Gene | 3718 (Hs), 16453 (Mm), 25326 (Rn) |
| Human Protein Atlas | ENSG00000105639 (Hs) |
| KEGG Enzyme | 2.7.10.2 |
| KEGG Gene | hsa:3718 (Hs), mmu:16453 (Mm), rno:25326 (Rn) |
| OMIM | 600173 (Hs) |
| Orphanet | ORPHA122729 (Hs) |
| Pharos | P52333 (Hs) |
| RefSeq Nucleotide | NM_000215 (Hs), NM_001190830 (Mm), NM_012855 (Rn) |
| RefSeq Protein | NP_000206 (Hs), NP_001177759 (Mm), NP_036987 (Rn) |
| SynPHARM |
80437 (in complex with JAK inhibitor I) 85283 (in complex with tofacitinib) |
| UniProtKB | P52333 (Hs), Q62137 (Mm), Q63272 (Rn) |
| Wikipedia | JAK3 (Hs) |
Selected 3D Structures  |
|||||||||||||
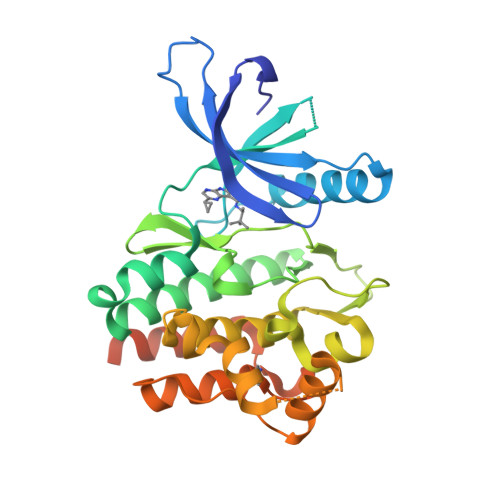
|
|
||||||||||||
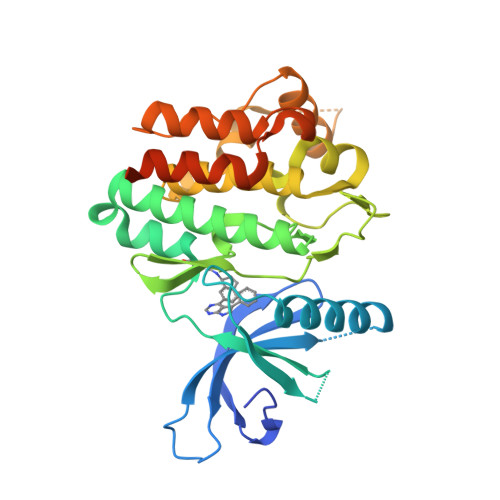
|
|
||||||||||||
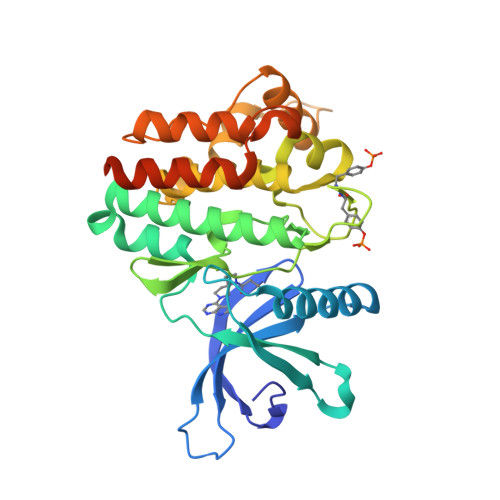
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inhibitor Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The IC50 for filgotinib vs. JAK3 is >10 μM [17]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other Binding Ligands | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 19,57 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: JAK3(JH1domain-catalytic) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: 2,26 |

|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: JAK3/JAK3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| Psoriatic skin samples show elevated JAK3 (and JAK1) expression, with signalling predominantly through STAT3 [1]. JAK3 upregulation is observed in skin cells and dermal inflammatory infiltrate. Noncoding mutation which creates a dominant splice site and reduces wild-type JAK3 protein expression is the cause of SCID/CID in siblings [44]. |
| Immuno Process Associations | |||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
Biologically Significant Variants 
|
||||||||||||
|
||||||||||||
|
1. Alves de Medeiros AK, Speeckaert R, Desmet E, Van Gele M, De Schepper S, Lambert J. (2016) JAK3 as an Emerging Target for Topical Treatment of Inflammatory Skin Diseases. PLoS ONE, 11 (10): e0164080. [PMID:27711196]
2. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377]
3. Anderson DR, Hockerman SL, Blinn JR, Jacobsen EJ. (2019) Substituted pyrrolopyrimidine jak inhibitors and methods of making and using the same. Patent number: WO2019090158A1. Assignee: Aclaris Therapeutics, Inc.. Priority date: 02/10/2029. Publication date: 02/08/2029.
4. Asshoff M, Petzer V, Warr MR, Haschka D, Tymoszuk P, Demetz E, Seifert M, Posch W, Nairz M, Maciejewski P et al.. (2017) Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood, 129 (13): 1823-1830. [PMID:28188131]
5. Bach J, Eastwood P, González J, Gómez E, Alonso JA, Fonquerna S, Lozoya E, Orellana A, Maldonado M, Calaf E et al.. (2019) Identification of 2-Imidazopyridine and 2-Aminopyridone Purinones as Potent Pan-Janus Kinase (JAK) Inhibitors for the Inhaled Treatment of Respiratory Diseases. J Med Chem, 62 (20): 9045-9060. [PMID:31609613]
6. Ban SA, Salzer E, Eibl MM, Linder A, Geier CB, Santos-Valente E, Garncarz W, Lion T, Ott R, Seelbach C et al.. (2014) Combined immunodeficiency evolving into predominant CD4+ lymphopenia caused by somatic chimerism in JAK3. J Clin Immunol, 34 (8): 941-53. [PMID:25205547]
7. Berlinski PJ, Birchmeier MJ, Bowman JW, Gonzales AJ, Kamerling SG, Mann DW, Mitton-Fry MJ. (2010) Pyrrolo[2,3-d]pyrimidine compounds. Patent number: WO2010020905. Assignee: Pfizer Inc.. Priority date: 20/08/2008. Publication date: 25/02/2010.
8. Blanc J. (2010) Novel compound useful for the treatment of degenerative and inflammatory diseases. Patent number: WO2010149771. Assignee: Galapagos Nv, Menet, Christel Jeanne Marie. Priority date: 26/06/2009. Publication date: 29/12/2010.
9. Brown GR, Bamford AM, Bowyer J, James DS, Rankine N, Tang E, Torr V, Culbert EJ. (2000) Naphthyl ketones: a new class of Janus kinase 3 inhibitors. Bioorg Med Chem Lett, 10 (6): 575-9. [PMID:10741557]
10. Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, Chaves J, Wierda WG, Awan FT, Brown JR et al.. (2016) Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med, 374 (4): 323-32. [PMID:26641137]
11. Casimiro-Garcia A, Trujillo JI, Vajdos F, Juba B, Banker ME, Aulabaugh A, Balbo P, Bauman J, Chrencik J, Coe JW et al.. (2018) Identification of Cyanamide-Based Janus Kinase 3 (JAK3) Covalent Inhibitors. J Med Chem, 61 (23): 10665-10699. [PMID:30423248]
12. Cha MY, Kang SJ, Kim MR, Lee JY, Jeon JY, Jo MG, Kwak EJ, Lee KO, Ha TH, Suh KH, Kim MS. (2011) Novel fused pyrimidine derivatives for inhd3ition of tyrosine kinase activity. Patent number: WO2011162515A2. Assignee: Hanmi Holdings Co. , Ltd.. Priority date: 23/06/2010. Publication date: 29/12/2011.
13. Chang Y, Min J, Jarusiewicz JA, Actis M, Yu-Chen Bradford S, Mayasundari A, Yang L, Chepyala D, Alcock LJ, Roberts KG et al.. (2021) Degradation of Janus kinases in CRLF2-rearranged acute lymphoblastic leukemia. Blood, 138 (23): 2313-2326. [PMID:34110416]
14. Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, Rizzuti BJ, Sawyer PS, Perry BD, Brissette WH et al.. (2003) Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science, 302 (5646): 875-8. [PMID:14593182]
15. Changelian PS, Moshinsky D, Kuhn CF, Flanagan ME, Munchhof MJ, Harris TM, Whipple DA, Doty JL, Sun J, Kent CR et al.. (2008) The specificity of JAK3 kinase inhibitors. Blood, 111 (4): 2155-7. [PMID:18094329]
16. Chen P, Norris D, Das J, Spergel SH, Wityak J, Leith L, Zhao R, Chen BC, Pitt S, Pang S et al.. (2004) Discovery of novel 2-(aminoheteroaryl)-thiazole-5-carboxamides as potent and orally active Src-family kinase p56(Lck) inhibitors. Bioorg Med Chem Lett, 14 (24): 6061-6. [PMID:15546730]
17. Clark JD, Flanagan ME, Telliez JB. (2014) Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem, 57 (12): 5023-38. [PMID:24417533]
18. Coffey G, Betz A, DeGuzman F, Pak Y, Inagaki M, Baker DC, Hollenbach SJ, Pandey A, Sinha U. (2014) The novel kinase inhibitor PRT062070 (Cerdulatinib) demonstrates efficacy in models of autoimmunity and B-cell cancer. J Pharmacol Exp Ther, 351 (3): 538-48. [PMID:25253883]
19. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
20. Dengler HS, Wu X, Peng I, Rinderknecht CH, Kwon Y, Suto E, Kohli PB, Liimatta M, Barrett K, Lloyd J et al.. (2018) Lung-restricted inhibition of Janus kinase 1 is effective in rodent models of asthma. Sci Transl Med, 10 (468): eaao2151. [PMID:30463918]
21. Dugan BJ, Gingrich DE, Mesaros EF, Milkiewicz KL, Curry MA, Zulli AL, Dobrzanski P, Serdikoff C, Jan M, Angeles TS et al.. (2012) A selective, orally bioavailable 1,2,4-triazolo[1,5-a]pyridine-based inhibitor of Janus kinase 2 for use in anticancer therapy: discovery of CEP-33779. J Med Chem, 55 (11): 5243-54. [PMID:22594690]
22. Elsayed MSA, Nielsen JJ, Park S, Park J, Liu Q, Kim CH, Pommier Y, Agama K, Low PS, Cushman M. (2018) Application of Sequential Palladium Catalysis for the Discovery of Janus Kinase Inhibitors in the Benzo[ c]pyrrolo[2,3- h][1,6]naphthyridin-5-one (BPN) Series. J Med Chem, 61 (23): 10440-10462. [PMID:30460842]
23. Fensome A, Ambler CM, Arnold E, Banker ME, Brown MF, Chrencik J, Clark JD, Dowty ME, Efremov IV, Flick A et al.. (2018) Dual Inhibition of TYK2 and JAK1 for the Treatment of Autoimmune Diseases: Discovery of (( S)-2,2-Difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl)amino)pyrimidin-4-yl)-3,8-diazabicyclo[3.2.1]octan-8-yl)methanone (PF-06700841). J Med Chem, 61 (19): 8597-8612. [PMID:30113844]
24. Flanagan ME, Blumenkopf TA, Brissette WH, Brown MF, Casavant JM, Shang-Poa C, Doty JL, Elliott EA, Fisher MB, Hines M et al.. (2010) Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem, 53 (24): 8468-84. [PMID:21105711]
25. Forsyth T, Kearney PC, Kim BG, Johnson HW, Aay N, Arcalas A, Brown DS, Chan V, Chen J, Du H et al.. (2012) SAR and in vivo evaluation of 4-aryl-2-aminoalkylpyrimidines as potent and selective Janus kinase 2 (JAK2) inhibitors. Bioorg Med Chem Lett, 22 (24): 7653-8. [PMID:23127890]
26. Gao Y, Davies SP, Augustin M, Woodward A, Patel UA, Kovelman R, Harvey KJ. (2013) A broad activity screen in support of a chemogenomic map for kinase signalling research and drug discovery. Biochem J, 451 (2): 313-28. [PMID:23398362]
27. Gozgit JM, Bebernitz G, Patil P, Ye M, Parmentier J, Wu J, Su N, Wang T, Ioannidis S, Davies A et al.. (2008) Effects of the JAK2 inhibitor, AZ960, on Pim/BAD/BCL-xL survival signaling in the human JAK2 V617F cell line SET-2. J Biol Chem, 283 (47): 32334-43. [PMID:18775810]
28. Hanan EJ, Liang J, Wang X, Blake RA, Blaquiere N, Staben ST. (2020) Monomeric Targeted Protein Degraders. J Med Chem, 63 (20): 11330-11361. [PMID:32352776]
29. Hanan EJ, van Abbema A, Barrett K, Blair WS, Blaney J, Chang C, Eigenbrot C, Flynn S, Gibbons P, Hurley CA et al.. (2012) Discovery of potent and selective pyrazolopyrimidine janus kinase 2 inhibitors. J Med Chem, 55 (22): 10090-107. [PMID:23061660]
30. Howard S, Berdini V, Boulstridge JA, Carr MG, Cross DM, Curry J, Devine LA, Early TR, Fazal L, Gill AL et al.. (2009) Fragment-based discovery of the pyrazol-4-yl urea (AT9283), a multitargeted kinase inhibitor with potent aurora kinase activity. J Med Chem, 52 (2): 379-88. [PMID:19143567]
31. Hudson R, Kozak J, Fatheree PR, Podesto DD, Brandt GEL, Fleury M, Beausoleil A-M, Huang X, Thalladi VR. (2016) Naphthyridine compounds as jak kinase inhibitors. Patent number: WO2016191524A1. Assignee: Theravance Biopharma R&D. Priority date: 28/05/2015. Publication date: 01/12/2016.
32. Ito M, Yamazaki S, Yamagami K, Kuno M, Morita Y, Okuma K, Nakamura K, Chida N, Inami M, Inoue T et al.. (2017) A novel JAK inhibitor, peficitinib, demonstrates potent efficacy in a rat adjuvant-induced arthritis model. J Pharmacol Sci, 133 (1): 25-33. [PMID:28117214]
33. Jones P, Storer RI, Sabnis YA, Wakenhut FM, Whitlock GA, England KS, Mukaiyama T, Dehnhardt CM, Coe JW, Kortum SW et al.. (2017) Design and Synthesis of a Pan-Janus Kinase Inhibitor Clinical Candidate (PF-06263276) Suitable for Inhaled and Topical Delivery for the Treatment of Inflammatory Diseases of the Lungs and Skin. J Med Chem, 60 (2): 767-786. [PMID:27983835]
34. Koudriakova T, Kreutter K, Leonard K, Rizzolio M, Smith RC, Tichenor MS, Wang A. (2018) Small molecule inhibitors of the JAK family of kinases. Patent number: WO2018112379A1. Assignee: Janssen Pharmaceutica. Priority date: 16/12/2016. Publication date: 21/06/2018.
35. Lam B, Arikawa Y, Cramlett J, Dong Q, de Jong R, Feher V, Grimshaw CE, Farrell PJ, Hoffman ID, Jennings A et al.. (2016) Discovery of TAK-659 an orally available investigational inhibitor of Spleen Tyrosine Kinase (SYK). Bioorg Med Chem Lett, 26 (24): 5947-5950. [PMID:27839918]
36. Liang X, Xie Y, Liu X, Xu H, Ren H, Tang S, Liu Q, Huang M, Shao X, Li C et al.. (2022) Discovery of Novel Imidazo[4,5-c]quinoline Derivatives to Treat Inflammatory Bowel Disease (IBD) by Inhibiting Multiple Proinflammatory Signaling Pathways and Restoring Intestinal Homeostasis. J Med Chem, 65 (18): 11949-11969. [PMID:36053746]
37. Long DD, Smith C, Thompson C. (2020) DIMETHYL AMINO AZETIDINE AMIDES AS JAK INHIBITORS. Patent number: WO/2020/051105. Assignee: THERAVANCE BIOPHARMA. Priority date: 04/09/2018. Publication date: 12/03/2020.
38. Ma L, Clayton JR, Walgren RA, Zhao B, Evans RJ, Smith MC, Heinz-Taheny KM, Kreklau EL, Bloem L, Pitou C et al.. (2013) Discovery and characterization of LY2784544, a small-molecule tyrosine kinase inhibitor of JAK2V617F. Blood Cancer J, 3: e109. [PMID:23584399]
39. Malerich JP, Lam JS, Hart B, Fine RM, Klebansky B, Tanga MJ, D'Andrea A. (2010) Diamino-1,2,4-triazole derivatives are selective inhibitors of TYK2 and JAK1 over JAK2 and JAK3. Bioorg Med Chem Lett, 20 (24): 7454-7. [PMID:21106455]
40. Nakaya Y, Shide K, Niwa T, Homan J, Sugahara S, Horio T, Kuramoto K, Kotera T, Shibayama H, Hori K et al.. (2011) Efficacy of NS-018, a potent and selective JAK2/Src inhibitor, in primary cells and mouse models of myeloproliferative neoplasms. Blood Cancer J, 1 (7): e29. [PMID:22829185]
41. Noji S, Hara Y, Miura T, Yamanaka H, Maeda K, Hori A, Yamamoto H, Obika S, Inoue M, Hase Y et al.. (2020) Discovery of a Janus Kinase Inhibitor Bearing a Highly Three-Dimensional Spiro Scaffold: JTE-052 (Delgocitinib) as a New Dermatological Agent to Treat Inflammatory Skin Disorders. J Med Chem, 63 (13): 7163-7185. [PMID:32511913]
42. Ostrovskyi D, Rumpf T, Eib J, Lumbroso A, Slynko I, Klaeger S, Heinzlmeir S, Forster M, Gehringer M, Pfaffenrot E et al.. (2016) Tofacitinib and analogs as inhibitors of the histone kinase PRK1 (PKN1). Future Med Chem, 8 (13): 1537-51. [PMID:27572962]
43. Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. (2009) CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia, 23 (8): 1441-5. [PMID:19295546]
44. Platt CD, Massaad MJ, Cangemi B, Schmidt B, Aldhekri H, Geha RS. (2017) Janus kinase 3 deficiency caused by a homozygous synonymous exonic mutation that creates a dominant splice site. J Allergy Clin Immunol, 140 (1): 268-271.e6. [PMID:27956217]
45. Purandare AV, McDevitt TM, Wan H, You D, Penhallow B, Han X, Vuppugalla R, Zhang Y, Ruepp SU, Trainor GL et al.. (2012) Characterization of BMS-911543, a functionally selective small-molecule inhibitor of JAK2. Leukemia, 26 (2): 280-8. [PMID:22015772]
46. Qiu Q, Chi F, Zhou D, Xie Z, Liu Y, Wu H, Yin Z, Shi W, Qian H. (2023) Exploration of Janus Kinase (JAK) and Histone Deacetylase (HDAC) Bispecific Inhibitors Based on the Moiety of Fedratinib for Treatment of Both Hematologic Malignancies and Solid Cancers. J Med Chem, 66 (8): 5753-5773. [PMID:37057760]
47. Soth M, Hermann JC, Yee C, Alam M, Barnett JW, Berry P, Browner MF, Frank K, Frauchiger S, Harris S et al.. (2013) 3-Amido pyrrolopyrazine JAK kinase inhibitors: development of a JAK3 vs JAK1 selective inhibitor and evaluation in cellular and in vivo models. J Med Chem, 56 (1): 345-56. [PMID:23214979]
48. Su Q, Ioannidis S, Chuaqui C, Almeida L, Alimzhanov M, Bebernitz G, Bell K, Block M, Howard T, Huang S et al.. (2014) Discovery of 1-methyl-1H-imidazole derivatives as potent Jak2 inhibitors. J Med Chem, 57 (1): 144-58. [PMID:24359159]
49. Suh B-C, Salgaonkar PD, Lee J, Koh JS, Song H-J, Lee IY, Lee J, Jung DS, Kim J-H, Kim S-W. (2016) Compounds and compositions for modulating EGFR mutant kinase activities. Patent number: WO2016060443A2. Assignee: Yuhan Corporation. Priority date: 13/10/2014. Publication date: 21/04/2016.
50. Tanimoto A, Ogawa Y, Oki C, Kimoto Y, Nozawa K, Amano W, Noji S, Shiozaki M, Matsuo A, Shinozaki Y et al.. (2015) Pharmacological properties of JTE-052: a novel potent JAK inhibitor that suppresses various inflammatory responses in vitro and in vivo. Inflamm Res, 64 (1): 41-51. [PMID:25387665]
51. Thoma G, Duthaler RO, Waelchli R, Hauchard A, Bruno S, Strittmatter-Keller U, Orjuela Leon A, Viebrock S, Aichholz R, Beltz K et al.. (2023) Discovery and Characterization of the Topical Soft JAK Inhibitor CEE321 for Atopic Dermatitis. J Med Chem, 66 (3): 2161-2168. [PMID:36657024]
52. Thompson JE, Cubbon RM, Cummings RT, Wicker LS, Frankshun R, Cunningham BR, Cameron PM, Meinke PT, Liverton N, Weng Y et al.. (2002) Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg Med Chem Lett, 12 (8): 1219-23. [PMID:11934592]
53. Van Rompaey L, Galien R, van der Aar EM, Clement-Lacroix P, Nelles L, Smets B, Lepescheux L, Christophe T, Conrath K, Vandeghinste N et al.. (2013) Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J Immunol, 191 (7): 3568-77. [PMID:24006460]
54. Voss JW, Camp HS, Padley RJ. (2015) Jak1 selective inhibitor and uses thereof. Patent number: WO2015061665. Assignee: Abbvie Inc.. Priority date: 24/10/2013. Publication date: 30/04/2015.
55. Wang T, Ioannidis S, Almeida L, Block MH, Davies AM, Lamb ML, Scott DA, Su M, Zhang HJ, Alimzhanov M et al.. (2011) In vitro and in vivo evaluation of 6-aminopyrazolyl-pyridine-3-carbonitriles as JAK2 kinase inhibitors. Bioorg Med Chem Lett, 21 (10): 2958-61. [PMID:21493067]
56. William AD, Lee AC, Blanchard S, Poulsen A, Teo EL, Nagaraj H, Tan E, Chen D, Williams M, Sun ET et al.. (2011) Discovery of the macrocycle 11-(2-pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a potent Janus kinase 2/fms-like tyrosine kinase-3 (JAK2/FLT3) inhibitor for the treatment of myelofibrosis and lymphoma. J Med Chem, 54 (13): 4638-58. [PMID:21604762]
57. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
58. Works MG, Yin F, Yin CC, Yiu Y, Shew K, Tran TT, Dunlap N, Lam J, Mitchell T, Reader J et al.. (2014) Inhibition of TYK2 and JAK1 ameliorates imiquimod-induced psoriasis-like dermatitis by inhibiting IL-22 and the IL-23/IL-17 axis. J Immunol, 193 (7): 3278-87. [PMID:25156366]
59. Xu X, Mao L, Xu W, Tang W, Zhang X, Xi B, Xu R, Fang X, Liu J, Fang C et al.. (2016) AC0010, an Irreversible EGFR Inhibitor Selectively Targeting Mutated EGFR and Overcoming T790M-Induced Resistance in Animal Models and Lung Cancer Patients. Mol Cancer Ther, 15 (11): 2586-2597. [PMID:27573423]
60. Yang T, Hu M, Chen Y, Xiang M, Tang M, Qi W, Shi M, He J, Yuan X, Zhang C et al.. (2020) N-(Pyrimidin-2-yl)-1,2,3,4-tetrahydroisoquinolin-6-amine Derivatives as Selective Janus Kinase 2 Inhibitors for the Treatment of Myeloproliferative Neoplasms. J Med Chem, 63 (23): 14921-14936. [PMID:33256400]
61. Yang T, Hu M, Qi W, Yang Z, Tang M, He J, Chen Y, Bai P, Yuan X, Zhang C et al.. (2019) Discovery of Potent and Orally Effective Dual Janus Kinase 2/FLT3 Inhibitors for the Treatment of Acute Myelogenous Leukemia and Myeloproliferative Neoplasms. J Med Chem, 62 (22): 10305-10320. [PMID:31670517]
62. Zhao C, Zhang Y, Zhang J, Li S, Liu M, Geng Y, Liu F, Chai Q, Meng H, Li M et al.. (2023) Discovery of Novel Fedratinib-Based HDAC/JAK/BRD4 Triple Inhibitors with Remarkable Antitumor Activity against Triple Negative Breast Cancer. J Med Chem, 66 (20): 14150-14174. [PMID:37796543]
63. Zhong Y, Dong S, Strattan E, Ren L, Butchar JP, Thornton K, Mishra A, Porcu P, Bradshaw JM, Bisconte A et al.. (2015) Targeting interleukin-2-inducible T-cell kinase (ITK) and resting lymphocyte kinase (RLK) using a novel covalent inhibitor PRN694. J Biol Chem, 290 (10): 5960-78. [PMID:25593320]
64. Zhu Y, Ma Y, Zu W, Song J, Wang H, Zhong Y, Li H, Zhang Y, Gao Q, Kong B et al.. (2020) Identification of N-Phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine Derivatives as Novel, Potent, and Selective NF-κB Inducing Kinase (NIK) Inhibitors for the Treatment of Psoriasis. J Med Chem, 63 (13): 6748-6773. [PMID:32479083]
Janus kinase (JakA) family: Janus kinase 3. Last modified on 06/10/2023. Accessed on 02/07/2025. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2049.