
Top ▲

GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 477 | 10q25.3 | ADRB1 | adrenoceptor beta 1 | 29 |
| Mouse | 7 | 466 | 19 51.96 cM | Adrb1 | adrenergic receptor, beta 1 | 36 |
| Rat | 7 | 466 | 1q55 | Adrb1 | adrenoceptor beta 1 | 52 |
Previous and Unofficial Names  |
| ADRB1R | Adrenergic receptor beta 1 | B1AR | beta-1 adrenergic receptor | beta-1 adrenoreceptor | Adrb-1 | beta 1-AR | adrenergic receptor |
Database Links  |
|
| Specialist databases | |
| GPCRdb | adrb1_human (Hs), adrb1_mouse (Mm), adrb1_rat (Rn) |
| Other databases | |
| Alphafold | P08588 (Hs), P34971 (Mm), P18090 (Rn) |
| ChEMBL Target | CHEMBL213 (Hs), CHEMBL3440 (Mm), CHEMBL3252 (Rn) |
| DrugBank Target | P08588 (Hs) |
| Ensembl Gene | ENSG00000043591 (Hs), ENSMUSG00000035283 (Mm), ENSRNOG00000017002 (Rn) |
| Entrez Gene | 153 (Hs), 11554 (Mm), 24925 (Rn) |
| Human Protein Atlas | ENSG00000043591 (Hs) |
| KEGG Gene | hsa:153 (Hs), mmu:11554 (Mm), rno:24925 (Rn) |
| OMIM | 109630 (Hs) |
| Pharos | P08588 (Hs) |
| RefSeq Nucleotide | NM_000684 (Hs), NM_007419 (Mm), NM_012701 (Rn) |
| RefSeq Protein | NP_000675 (Hs), NP_031445 (Mm), NP_036833 (Rn) |
| UniProtKB | P08588 (Hs), P34971 (Mm), P18090 (Rn) |
| Wikipedia | ADRB1 (Hs) |
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
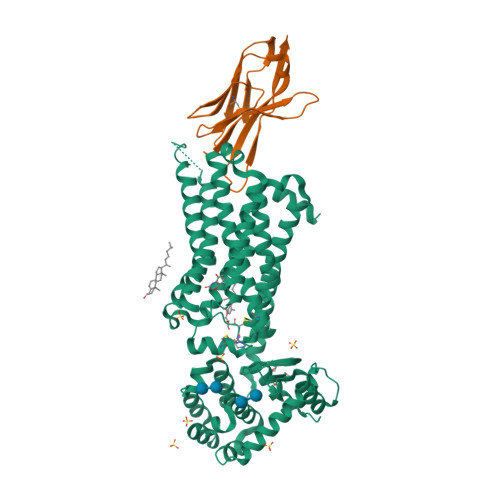
|
|
||||||||||||
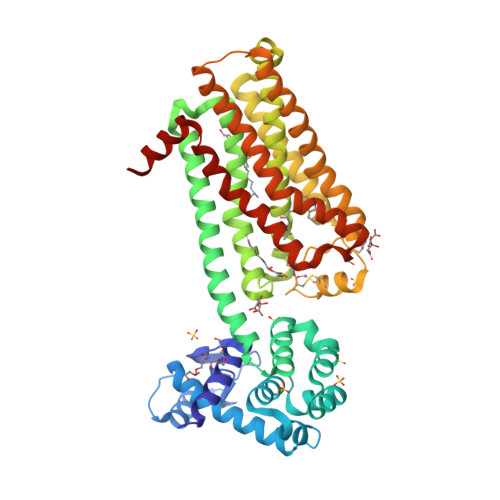
|
|
||||||||||||
Associated Proteins  |
|||||||||||||||
|
|||||||||||||||
Natural/Endogenous Ligands  |
| (-)-adrenaline |
| noradrenaline |
| (-)-noradrenaline |
| Comments: Noradrenaline exhibits greater potency than adrenaline |
| Potency order of endogenous ligands (Human) |
| (-)-noradrenaline > (-)-adrenaline |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BI-167107 is a high affinity β1-/β2-AR agonist that has been used to determine agonist bound structures of these β-subtypes [90]. RO-363 is the only β-AR agonist that displays significant selectivity for the β1-AR subtype and has been used experimentally to examine the physiological roles of β1-AR. ICI89406 and LK204-545 are partial agonists with significant β1-AR selectivity [58]. Noradrenaline displays a minor degree of selectivity for β1-AR and is used clinically by slow intravenous infusion to increase the blood pressure associated with shock mainly utilising its actions on α1-AR although the actions on β-AR may help maintain cardiac output. Xamoterol was trialled for the treatment of heart failure being a partial agonist that provided cardiac stimulation yet could block the deleterious effects of high plasma levels of endogenous catecholamines associated with this condition but unfortunately, prolonged stimulation of β1-AR with this compound clearly worsened heart failure and increased mortality so this approach was not successful. Xamoterol, denopamine and dobutamine have varying degrees of β1-AR selectivity and were previously used over short periods to maintain cardiac function in failure. Current clinical uses: noradrenaline and adrenaline are used clinically by slow intravenous infusion to increase the blood pressure associated with shock mainly utilising its actions on α1-AR although the actions on β-AR may help maintain cardiac output. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CGP 12177 is listed as a non-selective partial agonist at the β1-AR. It has now been established that the agonist action of this ligand is a result of action at a non-catecholamine activated site on the β1-AR [11]. This site is resistant to propranolol but is eliminated in β1-AR knockout mice, confirming the site of action as the β1-AR. This site was previously referred to as the β4-AR [38]. At the β1-AR, CGP12177 is a high affinity antagonist of the orthosteric catecholamine conformation. At higher concentrations, it activates a secondary conformation of the β1-AR. This secondary conformation can be activated by several compounds including pindolol, alprenolol, cyanopindolol, carazolol and carvedilol and is relatively resistant to antagonism by many β-antagonists (compared to the catecholamine conformation). Many of the antagonists acting at β1-AR display partial agonist properties including pindolol, carazolol, labetalol, SR59230A, LK 204-545, cyanopindolol, carvedilol and acebutolol [6-7,9] particularly in in vitro assays. Propafenone also primarily blocks α-subunits of sodium ion channels (see the Voltage-gated sodium channels family in the Ion Channels section of this website for further details). Cyanopindolol and 7-methyl cyanopindolol are high affinity antagonists or very weak partial agonists that have been used to explore the relationship between structure and efficacy [73]. CGP20712A is the most selective β1-AR antagonist that is easily available and is widely used in in vitro studies with ≥1000 fold selectivity vs. the other 8 adrenoceptors but does have some off-target activity on other receptors. NDD-713 and NDD-825 are high-affinity, β1-AR selective ligands devoid of agonist activity, off-target effects, and toxicology issues, but with good distribution, metabolism and elimination properties. These ligands are largely devoid of the β2-AR-mediated adverse effects of bronchospasm and vasoconstriction and may be beneficial in patients with cardiovascular and respiratory disease or limb ischemia. Most so-called cardioselective β-AR antagonists display only modest selectivity for β1-AR. Current clinical uses: widely used for heart failure, ischaemic heart disease, cardiac arrythmias, hypertension, glaucoma, portal hypertension, anxiety, migraine, benign essential tremor, thyrotoxicosis, infantile haemangioma (some may target β2-AR as well as β1-AR). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| β1-AR is involved in immune regulation and inflammation. Inflammation: Astrocytes [15] and microglia [79]. Anti-β1-AR autoantibodies: effect on β1-AR conformation and function [19,26], in heart failure [18,28], cardiac fibrosis [51], and cardiomyopathy [51], |
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
| Gs family | Adenylyl cyclase stimulation |
| Comments: Stimulation of adenylate cyclase (AC) causes the conversion of ATP into cAMP. This activates protein kinase A, which in turn phosphorylates several substrates, for example L-type Ca2+ channels. | |
| References: 77,88 | |
Secondary Transduction Mechanisms  |
|
| Transducer | Effector/Response |
| Gi/Go family |
Guanylate cyclase stimulation Other - See Comments |
|
Comments:
ERK1/2 phosphorylation. Stimulation of guanylate cyclase (GC) causes an increase in cGMP levels, and subsequent activation of protein kinase G. |
|
| References: 49,84 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Biologically Significant Variants 
|
||||||||||
|
||||||||||
|
| General Comments |
| For a review on the β-adrenoceptor polymorphisms see reference [48]. |
1. Ahles A, Rodewald F, Rochais F, Bünemann M, Engelhardt S. (2015) Interhelical interaction and receptor phosphorylation regulate the activation kinetics of different human β1-adrenoceptor variants. J Biol Chem, 290 (3): 1760-9. [PMID:25451930]
2. André C, Erraji L, Gaston J, Grimber G, Briand P, Guillet JG. (1996) Transgenic mice carrying the human beta 2-adrenergic receptor gene with its own promoter overexpress beta 2-adrenergic receptors in liver. Eur J Biochem, 241 (2): 417-24. [PMID:8917438]
3. Aparici M, Gómez-Angelats M, Vilella D, Otal R, Carcasona C, Viñals M, Ramos I, Gavaldà A, De Alba J, Gras J et al.. (2012) Pharmacological characterization of abediterol, a novel inhaled β(2)-adrenoceptor agonist with long duration of action and a favorable safety profile in preclinical models. J Pharmacol Exp Ther, 342 (2): 497-509. [PMID:22588259]
4. Auerbach SS, DrugMatrix® and ToxFX® Coordinator National Toxicology Program. National Toxicology Program: Dept of Health and Human Services. Accessed on 02/05/2014. Modified on 02/05/2014. DrugMatrix, https://ntp.niehs.nih.gov/drugmatrix/index.html
5. Baker JG. (2005) The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol, 144 (3): 317-22. [PMID:15655528]
6. Baker JG. (2010) The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol, 160 (5): 1048-61. [PMID:20590599]
7. Baker JG, Gardiner SM, Woolard J, Fromont C, Jadhav GP, Mistry SN, Thompson KSJ, Kellam B, Hill SJ, Fischer PM. (2017) Novel selective β1-adrenoceptor antagonists for concomitant cardiovascular and respiratory disease. FASEB J, 31 (7): 3150-3166. [PMID:28400472]
8. Baker JG, Hall IP, Hill SJ. (2003) Agonist and inverse agonist actions of beta-blockers at the human beta 2-adrenoceptor provide evidence for agonist-directed signaling. Mol Pharmacol, 64 (6): 1357-69. [PMID:14645666]
9. Baker JG, Kemp P, March J, Fretwell L, Hill SJ, Gardiner SM. (2011) Predicting in vivo cardiovascular properties of β-blockers from cellular assays: a quantitative comparison of cellular and cardiovascular pharmacological responses. FASEB J, 25 (12): 4486-97. [PMID:21865315]
10. Baker JG, Proudman RG, Hill SJ. (2013) Impact of polymorphic variants on the molecular pharmacology of the two-agonist conformations of the human β1-adrenoceptor. PLoS One, 8 (11): e77582. [PMID:24250787]
11. Baker JG, Proudman RG, Hill SJ. (2014) Identification of key residues in transmembrane 4 responsible for the secondary, low-affinity conformation of the human β1-adrenoceptor. Mol Pharmacol, 85 (5): 811-29. [PMID:24608857]
12. Baker JG, Proudman RG, Hill SJ. (2015) Salmeterol's extreme β2 selectivity is due to residues in both extracellular loops and transmembrane domains. Mol Pharmacol, 87 (1): 103-20. [PMID:25324048]
13. Beattie D, Beer D, Bradley ME, Bruce I, Charlton SJ, Cuenoud BM, Fairhurst RA, Farr D, Fozard JR, Janus D et al.. (2012) An investigation into the structure-activity relationships associated with the systematic modification of the β(2)-adrenoceptor agonist indacaterol. Bioorg Med Chem Lett, 22 (19): 6280-5. [PMID:22932315]
14. Beattie D, Bradley M, Brearley A, Charlton SJ, Cuenoud BM, Fairhurst RA, Gedeck P, Gosling M, Janus D, Jones D et al.. (2010) A physical properties based approach for the exploration of a 4-hydroxybenzothiazolone series of beta2-adrenoceptor agonists as inhaled long-acting bronchodilators. Bioorg Med Chem Lett, 20 (17): 5302-7. [PMID:20655218]
15. Benton KC, Wheeler DS, Kurtoglu B, Ansari MBZ, Cibich DP, Gonzalez DA, Herbst MR, Khursheed S, Knorr RC, Lobner D et al.. (2022) Norepinephrine activates β1 -adrenergic receptors at the inner nuclear membrane in astrocytes. Glia, 70 (9): 1777-1794. [PMID:35589612]
16. Berthouze-Duquesnes M, Lucas A, Saulière A, Sin YY, Laurent AC, Galés C, Baillie G, Lezoualc'h F. (2013) Specific interactions between Epac1, β-arrestin2 and PDE4D5 regulate β-adrenergic receptor subtype differential effects on cardiac hypertrophic signaling. Cell Signal, 25 (4): 970-80. [PMID:23266473]
17. Bilezikian JP. (1987) Defining the Role of Adrenergic Receptors in Human Physiology. In Adrenergic Receptors in Man. Edited by Insel PA (Marcel Dekker) 37-68. [ISBN:0824776291]
18. Boivin-Jahns V, Uhland K, Holthoff HP, Beyersdorf N, Kocoski V, Kerkau T, Münch G, Lohse MJ, Ungerer M, Jahns R. (2018) Cyclopeptide COR-1 to treat beta1-adrenergic receptor antibody-induced heart failure. PLoS One, 13 (8): e0201160. [PMID:30125285]
19. Bornholz B, Weidtkamp-Peters S, Schmitmeier S, Seidel CA, Herda LR, Felix SB, Lemoine H, Hescheler J, Nguemo F, Schäfer C et al.. (2013) Impact of human autoantibodies on β1-adrenergic receptor conformation, activity, and internalization. Cardiovasc Res, 97 (3): 472-80. [PMID:23208588]
20. Bristow MR, Hershberger RE, Port JD, Minobe W, Rasmussen R. (1989) Beta 1- and beta 2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol Pharmacol, 35 (3): 295-303. [PMID:2564629]
21. Buxton BF, Jones CR, Molenaar P, Summers RJ. (1987) Characterization and autoradiographic localization of beta-adrenoceptor subtypes in human cardiac tissues. Br J Pharmacol, 92 (2): 299-310. [PMID:2823947]
22. Candelore MR, Deng L, Tota L, Guan XM, Amend A, Liu Y, Newbold R, Cascieri MA, Weber AE. (1999) Potent and selective human beta(3)-adrenergic receptor antagonists. J Pharmacol Exp Ther, 290 (2): 649-55. [PMID:10411574]
23. Chen L, Xiao T, Chen L, Xie S, Deng M, Wu D. (2018) The Association of ADRB1 and CYP2D6 Polymorphisms With Antihypertensive Effects and Analysis of Their Contribution to Hypertension Risk. Am J Med Sci, 355 (3): 235-239. [PMID:29549925]
24. Cros C, Brette F. (2013) Functional subcellular distribution of β1- and β2-adrenergic receptors in rat ventricular cardiac myocytes. Physiol Rep, 1 (3): e00038. [PMID:24303124]
25. Dehvari N, Sato M, Bokhari MH, Kalinovich A, Ham S, Gao J, Nguyen HTM, Whiting L, Mukaida S, Merlin J et al.. (2020) The metabolic effects of mirabegron are mediated primarily by β3 -adrenoceptors. Pharmacol Res Perspect, 8 (5): e00643. [PMID:32813332]
26. Du Y, Yan L, Wang J, Zhan W, Song K, Han X, Li X, Cao J, Liu H. (2012) β1-Adrenoceptor autoantibodies from DCM patients enhance the proliferation of T lymphocytes through the β1-AR/cAMP/PKA and p38 MAPK pathways. PLoS One, 7 (12): e52911. [PMID:23300817]
27. Elnatan J, Molenaar P, Rosenfeldt FL, Summers RJ. (1994) Autoradiographic localization and quantitation of beta 1- and beta 2-adrenoceptors in the human atrioventricular conducting system: a comparison of patients with idiopathic dilated cardiomyopathy and ischemic heart disease. J Mol Cell Cardiol, 26 (3): 313-23. [PMID:7913135]
28. Ernst D, Westerbergh J, Sogkas G, Jablonka A, Ahrenstorf G, Schmidt RE, Heidecke H, Wallentin L, Riemekasten G, Witte T. (2019) Lowered anti-beta1 adrenergic receptor antibody concentrations may have prognostic significance in acute coronary syndrome. Sci Rep, 9 (1): 14552. [PMID:31601947]
29. Frielle T, Collins S, Daniel KW, Caron MG, Lefkowitz RJ, Kobilka BK. (1987) Cloning of the cDNA for the human β1-adrenergic receptor. Proc Natl Acad Sci USA, 84: 7920-7924. [PMID:2825170]
30. Frielle T, Daniel KW, Caron MG, Lefkowitz RJ. (1988) Structural basis of beta-adrenergic receptor subtype specificity studied with chimeric beta 1/beta 2-adrenergic receptors. Proc Natl Acad Sci USA, 85 (24): 9494-8. [PMID:2849109]
31. Grisan F, Burdyga A, Iannucci LF, Surdo NC, Pozzan T, Di Benedetto G, Lefkimmiatis K. (2020) Studying β1 and β2 adrenergic receptor signals in cardiac cells using FRET-based sensors. Prog Biophys Mol Biol, 154: 30-38. [PMID:31266653]
32. Hancock AA, DeLean AL, Lefkowitz RJ. (1979) Quantitative resolution of beta-adrenergic receptor subtypes by selective ligand binding: application of a computerized model fitting technique. Mol Pharmacol, 16 (1): 1-9. [PMID:39239]
33. Hoenke C, Bouyssou T, Tautermann CS, Rudolf K, Schnapp A, Konetzki I. (2009) Use of 5-hydroxy-4H-benzo[1,4]oxazin-3-ones as beta2-adrenoceptor agonists. Bioorg Med Chem Lett, 19 (23): 6640-4. [PMID:19875286]
34. Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. (2004) Comparative pharmacology of human beta-adrenergic receptor subtypes--characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol, 369 (2): 151-9. [PMID:14730417]
35. Isogaya M, Sugimoto Y, Tanimura R, Tanaka R, Kikkawa H, Nagao T, Kurose H. (1999) Binding pockets of the beta(1)- and beta(2)-adrenergic receptors for subtype-selective agonists. Mol Pharmacol, 56 (5): 875-85. [PMID:10531390]
36. Jasper JR, Link RE, Chruscinski AJ, Kobilka BK, Bernstein D. (1993) Primary structure of the mouse beta 1-adrenergic receptor gene. Biochim Biophys Acta, 1178 (3): 307-9. [PMID:8395893]
37. Jeff JM, Donahue BS, Brown-Gentry K, Roden DM, Crawford DC, Stein CM, Kurnik D. (2014) Genetic variation in the β1-adrenergic receptor is associated with the risk of atrial fibrillation after cardiac surgery. Am Heart J, 167 (1): 101-108.e1. [PMID:24332148]
38. Joseph SS, Lynham JA, Colledge WH, Kaumann AJ. (2004) Binding of (-)-[3H]-CGP12177 at two sites in recombinant human beta 1-adrenoceptors and interaction with beta-blockers. Naunyn Schmiedebergs Arch Pharmacol, 369 (5): 525-32. [PMID:15060759]
39. Joseph SS, Lynham JA, Grace AA, Colledge WH, Kaumann AJ. (2004) Markedly reduced effects of (-)-isoprenaline but not of (-)-CGP12177 and unchanged affinity of beta-blockers at Gly389-beta1-adrenoceptors compared to Arg389-beta1-adrenoceptors. Br J Pharmacol, 142 (1): 51-6. [PMID:15037517]
40. Joseph SS, Lynham JA, Molenaar P, Grace AA, Colledge WH, Kaumann AJ. (2003) Intrinsic sympathomimetic activity of (-)-pindolol mediated through a (-)-propranolol-resistant site of the beta1-adrenoceptor in human atrium and recombinant receptors. Naunyn Schmiedebergs Arch Pharmacol, 368 (6): 496-503. [PMID:14608456]
41. Jung H, Windhaber R, Palm D, Schnackerz KD. (1995) NMR and circular dichroism studies of synthetic peptides derived from the third intracellular loop of the beta-adrenoceptor. FEBS Lett, 358 (2): 133-6. [PMID:7828722]
42. Kao DP, Davis G, Aleong R, O'Connor CM, Fiuzat M, Carson PE, Anand IS, Plehn JF, Gottlieb SS, Silver MA et al.. (2013) Effect of bucindolol on heart failure outcomes and heart rate response in patients with reduced ejection fraction heart failure and atrial fibrillation. Eur J Heart Fail, 15 (3): 324-33. [PMID:23223178]
43. Kompa AR, Summers RJ. (1999) Desensitization and resensitization of beta 1- and putative beta 4-adrenoceptor mediated responses occur in parallel in a rat model of cardiac failure. Br J Pharmacol, 128 (7): 1399-406. [PMID:10602318]
44. Krushinski Jr JH, Schaus JM, Thompson DC, Calligaro DO, Nelson DL, Luecke SH, Wainscott DB, Wong DT. (2007) Indoloxypropanolamine analogues as 5-HT(1A) receptor antagonists. Bioorg Med Chem Lett, 17 (20): 5600-4. [PMID:17804228]
45. Lavoie C, Hébert TE. (2003) Pharmacological characterization of putative beta1-beta2-adrenergic receptor heterodimers. Can J Physiol Pharmacol, 81 (2): 186-95. [PMID:12710533]
46. Lavoie C, Mercier JF, Salahpour A, Umapathy D, Breit A, Villeneuve LR, Zhu WZ, Xiao RP, Lakatta EG, Bouvier M et al.. (2002) Beta 1/beta 2-adrenergic receptor heterodimerization regulates beta 2-adrenergic receptor internalization and ERK signaling efficacy. J Biol Chem, 277 (38): 35402-10. [PMID:12140284]
47. Lee YH, Petkova AP, Konkar AA, Granneman JG. (2015) Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J, 29 (1): 286-99. [PMID:25392270]
48. Leineweber K, Büscher R, Bruck H, Brodde OE. (2004) Beta-adrenoceptor polymorphisms. Naunyn Schmiedebergs Arch Pharmacol, 369 (1): 1-22. [PMID:14647973]
49. Li F, De Godoy M, Rattan S. (2004) Role of adenylate and guanylate cyclases in beta1-, beta2-, and beta3-adrenoceptor-mediated relaxation of internal anal sphincter smooth muscle. J Pharmacol Exp Ther, 308 (3): 1111-20. [PMID:14711933]
50. Louis SN, Nero TL, Iakovidis D, Jackman GP, Louis WJ. (1999) LK 204-545, a highly selective beta1-adrenoceptor antagonist at human beta-adrenoceptors. Eur J Pharmacol, 367 (2-3): 431-5. [PMID:10079020]
51. Lv T, Du Y, Cao N, Zhang S, Gong Y, Bai Y, Wang W, Liu H. (2016) Proliferation in cardiac fibroblasts induced by β1-adrenoceptor autoantibody and the underlying mechanisms. Sci Rep, 6: 32430. [PMID:27577254]
52. Machida CA, Bunzow JR, Searles RP, Van Tol H, Tester B, Neve KA, Teal P, Nipper V, Civelli O. (1990) Molecular cloning and expression of the rat beta 1-adrenergic receptor gene. J Biol Chem, 265 (22): 12960-5. [PMID:1695899]
53. Maqbool A, Hall AS, Ball SG, Balmforth AJ. (1999) Common polymorphisms of beta1-adrenoceptor: identification and rapid screening assay. Lancet, 353 (9156): 897. [PMID:10093986]
54. Mason DA, Moore JD, Green SA, Liggett SB. (1999) A gain-of-function polymorphism in a G-protein coupling domain of the human beta1-adrenergic receptor. J Biol Chem, 274 (18): 12670-4. [PMID:10212248]
55. Mercier JF, Salahpour A, Angers S, Breit A, Bouvier M. (2002) Quantitative assessment of beta 1- and beta 2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J Biol Chem, 277 (47): 44925-31. [PMID:12244098]
56. Miller-Gallacher JL, Nehmé R, Warne T, Edwards PC, Schertler GF, Leslie AG, Tate CG. (2014) The 2.1 Å resolution structure of cyanopindolol-bound β1-adrenoceptor identifies an intramembrane Na+ ion that stabilises the ligand-free receptor. PLoS One, 9 (3): e92727. [PMID:24663151]
57. Minneman KP, Hegstrand LR, Molinoff PB. (1979) The pharmacological specificity of beta-1 and beta-2 adrenergic receptors in rat heart and lung in vitro. Mol Pharmacol, 16 (1): 21-33. [PMID:39243]
58. Mistry SN, Baker JG, Fischer PM, Hill SJ, Gardiner SM, Kellam B. (2013) Synthesis and in vitro and in vivo characterization of highly β1-selective β-adrenoceptor partial agonists. J Med Chem, 56 (10): 3852-65. [PMID:23614528]
59. Molenaar P, Sarsero D, Arch JR, Kelly J, Henson SM, Kaumann AJ. (1997) Effects of (-)-RO363 at human atrial beta-adrenoceptor subtypes, the human cloned beta 3-adrenoceptor and rodent intestinal beta 3-adrenoceptors. Br J Pharmacol, 120 (2): 165-76. [PMID:9117106]
60. Molenaar P, Savarimuthu SM, Sarsero D, Chen L, Semmler AB, Carle A, Yang I, Bartel S, Vetter D, Beyerdörfer I et al.. (2007) (-)-Adrenaline elicits positive inotropic, lusitropic, and biochemical effects through beta2 -adrenoceptors in human atrial myocardium from nonfailing and failing hearts, consistent with Gs coupling but not with Gi coupling. Naunyn Schmiedebergs Arch Pharmacol, 375 (1): 11-28. [PMID:17295024]
61. Moukhametzianov R, Warne T, Edwards PC, Serrano-Vega MJ, Leslie AG, Tate CG, Schertler GF. (2011) Two distinct conformations of helix 6 observed in antagonist-bound structures of a beta1-adrenergic receptor. Proc Natl Acad Sci USA, 108 (20): 8228-32. [PMID:21540331]
62. Nasrollahi-Shirazi S, Sucic S, Yang Q, Freissmuth M, Nanoff C. (2016) Comparison of the β-Adrenergic Receptor Antagonists Landiolol and Esmolol: Receptor Selectivity, Partial Agonism, and Pharmacochaperoning Actions. J Pharmacol Exp Ther, 359 (1): 73-81. [PMID:27451411]
63. Obara K, Shigematsu M, Takahasi H, Iiboshi Y, Yoshioka K, Kasuya Y, Tanaka Y. (2020) Pharmacological properties of β-adrenoceptors mediating rat superior mesenteric artery relaxation and the effects of chemical sympathetic denervation. Life Sci, 241: 117155. [PMID:31837330]
64. Oostendorp J, Preitner F, Moffatt J, Jimenez M, Giacobino JP, Molenaar P, Kaumann AJ. (2000) Contribution of beta-adrenoceptor subtypes to relaxation of colon and oesophagus and pacemaker activity of ureter in wildtype and beta(3)-adrenoceptor knockout mice. Br J Pharmacol, 130 (4): 747-58. [PMID:10864880]
65. Pauwels PJ, Gommeren W, Van Lommen G, Janssen PA, Leysen JE. (1988) The receptor binding profile of the new antihypertensive agent nebivolol and its stereoisomers compared with various beta-adrenergic blockers. Mol Pharmacol, 34 (6): 843-51. [PMID:2462161]
66. Pönicke K, Heinroth-Hoffmann I, Brodde OE. (2003) Role of beta 1- and beta 2-adrenoceptors in hypertrophic and apoptotic effects of noradrenaline and adrenaline in adult rat ventricular cardiomyocytes. Naunyn Schmiedebergs Arch Pharmacol, 367 (6): 592-9. [PMID:12750877]
67. Ranade K, Jorgenson E, Sheu WH, Pei D, Hsiung CA, Chiang FT, Chen YD, Pratt R, Olshen RA, Curb D et al.. (2002) A polymorphism in the beta1 adrenergic receptor is associated with resting heart rate. Am J Hum Genet, 70 (4): 935-42. [PMID:11854867]
68. Roberts SJ, Papaioannou M, Evans BA, Summers RJ. (1997) Functional and molecular evidence for beta 1-, beta 2- and beta 3- adrenoceptors in human colon. Br J Pharmacol, 120 (8): 1527-35. [PMID:9113375]
69. Roberts SJ, Papaioannou M, Evans BA, Summers RJ. (1999) Characterization of beta-adrenoceptor mediated smooth muscle relaxation and the detection of mRNA for beta1-, beta2- and beta3-adrenoceptors in rat ileum. Br J Pharmacol, 127 (4): 949-61. [PMID:10433503]
70. Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK. (1999) Cardiovascular and metabolic alterations in mice lacking both beta1- and beta2-adrenergic receptors. J Biol Chem, 274 (24): 16701-8. [PMID:10358009]
71. Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula Jr DP, Barsh GS, Bernstein D, Kobilka BK. (1996) Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci USA, 93 (14): 7375-80. [PMID:8693001]
72. Rohrer DK, Schauble EH, Desai KH, Kobilka BK, Bernstein D. (1998) Alterations in dynamic heart rate control in the beta 1-adrenergic receptor knockout mouse. Am J Physiol, 274 (4): H1184-93. [PMID:9575921]
73. Sato T, Baker J, Warne T, Brown GA, Leslie AG, Congreve M, Tate CG. (2015) Pharmacological Analysis and Structure Determination of 7-Methylcyanopindolol-Bound β1-Adrenergic Receptor. Mol Pharmacol, 88 (6): 1024-34. [PMID:26385885]
74. Sato Y, Kurose H, Isogaya M, Nagao T. (1996) Molecular characterization of pharmacological properties of T-0509 for beta-adrenoceptors. Eur J Pharmacol, 315 (3): 363-7. [PMID:8982677]
75. Sharif NA, Xu SX, Crider JY, McLaughlin M, Davis TL. (2001) Levobetaxolol (Betaxon) and other beta-adrenergic antagonists: preclinical pharmacology, IOP-lowering activity and sites of action in human eyes. J Ocul Pharmacol Ther, 17 (4): 305-17. [PMID:11572462]
76. Soave M, Stoddart LA, Brown A, Woolard J, Hill SJ. (2016) Use of a new proximity assay (NanoBRET) to investigate the ligand-binding characteristics of three fluorescent ligands to the human β1-adrenoceptor expressed in HEK-293 cells. Pharmacol Res Perspect, 4 (5): e00250. [PMID:27588207]
77. Stiles GL, Caron MG, Lefkowitz RJ. (1984) Beta-adrenergic receptors: biochemical mechanisms of physiological regulation. Physiol Rev, 64 (2): 661-743. [PMID:6143332]
78. Su M, Zhu L, Zhang Y, Paknejad N, Dey R, Huang J, Lee MY, Williams D, Jordan KD, Eng ET et al.. (2020) Structural Basis of the Activation of Heterotrimeric Gs-Protein by Isoproterenol-Bound β1-Adrenergic Receptor. Mol Cell, 80 (1): 59-71.e4. [PMID:32818430]
79. Sugama S, Takenouchi T, Hashimoto M, Ohata H, Takenaka Y, Kakinuma Y. (2019) Stress-induced microglial activation occurs through β-adrenergic receptor: noradrenaline as a key neurotransmitter in microglial activation. J Neuroinflammation, 16 (1): 266. [PMID:31847911]
80. Summers RJ, Molnaar P, Russell F, Elnatan J, Jones CR, Buxton BF, Chang V, Hambley J. (1989) Coexistence and localization of beta 1- and beta 2-adrenoceptors in the human heart. Eur Heart J, 10 Suppl B: 11-21. [PMID:2553407]
81. Suzuki T, Nantel F, Bonin H, Valiquette M, Bouvier M. (1993) Cellular characterization of the pharmacological selectivity and tachyphylactic properties of denopamine for the human beta adrenergic receptors. J Pharmacol Exp Ther, 267 (2): 785-90. [PMID:7902433]
82. Takasu T, Ukai M, Sato S, Matsui T, Nagase I, Maruyama T, Sasamata M, Miyata K, Uchida H, Yamaguchi O. (2007) Effect of (R)-2-(2-aminothiazol-4-yl)-4'-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective beta3-adrenoceptor agonist, on bladder function. J Pharmacol Exp Ther, 321 (2): 642-7. [PMID:17293563]
83. Uehling DE, Shearer BG, Donaldson KH, Chao EY, Deaton DN, Adkison KK, Brown KK, Cariello NF, Faison WL, Lancaster ME et al.. (2006) Biarylaniline phenethanolamines as potent and selective beta3 adrenergic receptor agonists. J Med Chem, 49 (9): 2758-71. [PMID:16640337]
84. Wang J, Hanada K, Staus DP, Makara MA, Dahal GR, Chen Q, Ahles A, Engelhardt S, Rockman HA. (2017) Gαi is required for carvedilol-induced β1 adrenergic receptor β-arrestin biased signaling. Nat Commun, 8 (1): 1706. [PMID:29167435]
85. Wang Y, Shi Q, Li M, Zhao M, Reddy Gopireddy R, Teoh JP, Xu B, Zhu C, Ireton KE, Srinivasan S et al.. (2021) Intracellular β1-Adrenergic Receptors and Organic Cation Transporter 3 Mediate Phospholamban Phosphorylation to Enhance Cardiac Contractility. Circ Res, 128 (2): 246-261. [PMID:33183171]
86. Warne T, Moukhametzianov R, Baker JG, Nehmé R, Edwards PC, Leslie AG, Schertler GF, Tate CG. (2011) The structural basis for agonist and partial agonist action on a β(1)-adrenergic receptor. Nature, 469 (7329): 241-4. [PMID:21228877]
87. Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. (2008) Structure of a beta1-adrenergic G-protein-coupled receptor. Nature, 454 (7203): 486-91. [PMID:18594507]
88. Wenzel-Seifert K, Liu HY, Seifert R. (2002) Similarities and differences in the coupling of human beta1- and beta2-adrenoceptors to Gs(alpha) splice variants. Biochem Pharmacol, 64 (1): 9-20. [PMID:12106601]
89. Xu J, He J, Castleberry AM, Balasubramanian S, Lau AG, Hall RA. (2003) Heterodimerization of alpha 2A- and beta 1-adrenergic receptors. J Biol Chem, 278 (12): 10770-7. [PMID:12529373]
90. Xu X, Kaindl J, Clark MJ, Hübner H, Hirata K, Sunahara RK, Gmeiner P, Kobilka BK, Liu X. (2021) Binding pathway determines norepinephrine selectivity for the human β1AR over β2AR. Cell Res, 31 (5): 569-579. [PMID:33093660]
91. Yamada S, Niiya R, Ito Y, Kato Y, Onoue S. (2022) Comparative characterization of β-adrenoceptors in the bladder, heart, and lungs of rats: Alterations in spontaneously hypertensive rats. J Pharmacol Sci, 148 (1): 51-55. [PMID:34924129]
92. Yatani A, Tajima Y, Green SA. (1999) Coupling of beta-adrenergic receptors to cardiac L-type Ca2+ channels: preferential coupling of the beta1 versus beta2 receptor subtype and evidence for PKA-independent activation of the channel. Cell Signal, 11 (5): 337-42. [PMID:10376806]
93. Zhu WZ, Chakir K, Zhang S, Yang D, Lavoie C, Bouvier M, Hébert TE, Lakatta EG, Cheng H, Xiao RP. (2005) Heterodimerization of beta1- and beta2-adrenergic receptor subtypes optimizes beta-adrenergic modulation of cardiac contractility. Circ Res, 97 (3): 244-51. [PMID:16002745]